Blocking a vicious cycle nNOS/peroxynitrite/AMPK by S-nitrosoglutathione: implication for stroke therapy
- PMID: 26174015
- PMCID: PMC4502912
- DOI: 10.1186/s12868-015-0179-x
Blocking a vicious cycle nNOS/peroxynitrite/AMPK by S-nitrosoglutathione: implication for stroke therapy
Abstract
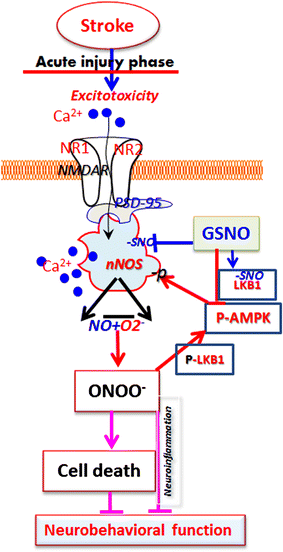
Background: Stroke immediately sets into motion sustained excitotoxicity and calcium dysregulation, causing aberrant activity in neuronal nitric oxide synthase (nNOS) and an imbalance in the levels of nitric oxide (NO). Drugs targeting nNOS-originated toxicity may therefore reduce stroke-induced damage. Recently, we observed that a redox-modulating agent of the NO metabolome, S-nitrosoglutathione (GSNO), confers neurovascular protection by reducing the levels of peroxynitrite, a product of aberrant NOS activity. We therefore investigated whether GSNO-mediated neuroprotection and improved neurological functions depend on blocking nNOS/peroxynitrite-associated injurious mechanisms using a rat model of cerebral ischemia reperfusion (IR).
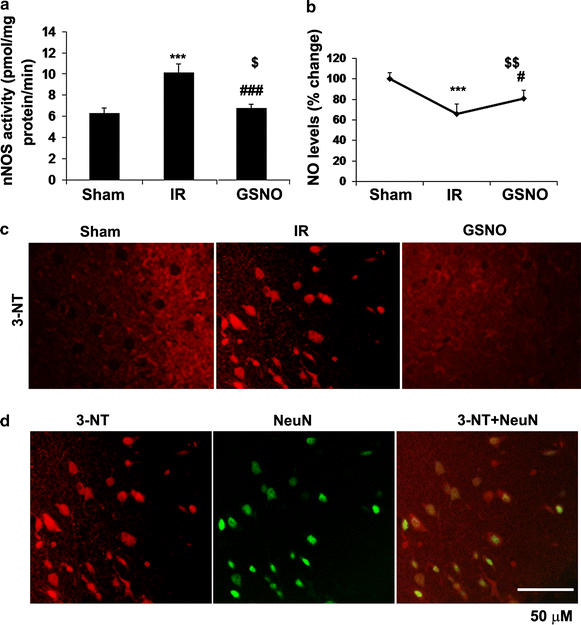
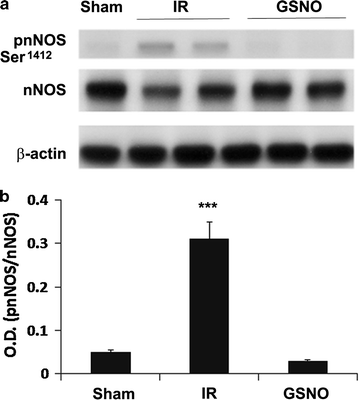
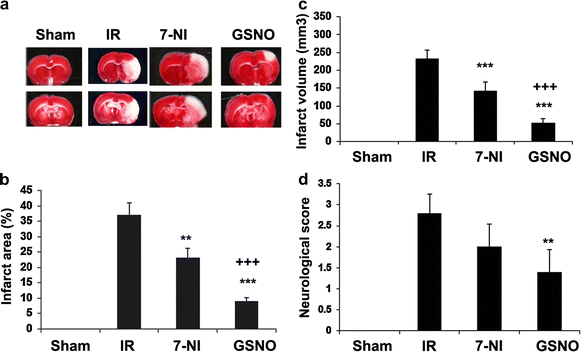
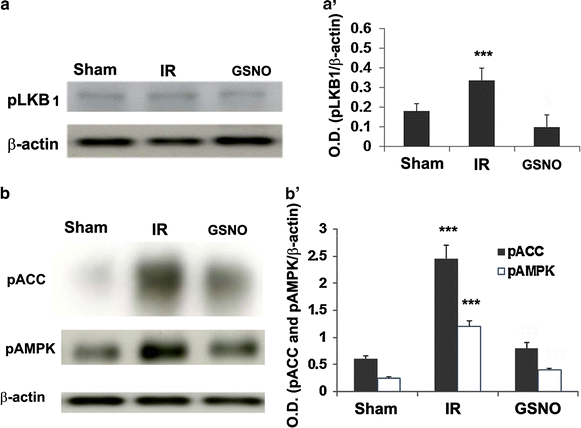
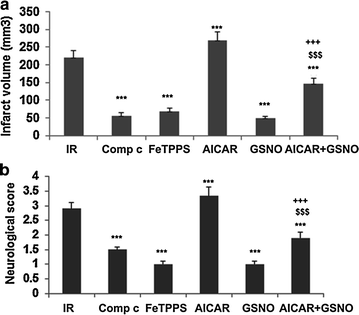
Results: IR increased the activity of nNOS, the levels of neuronal peroxynitrite and phosphorylation at Ser(1412) of nNOS. GSNO treatment of IR animals decreased IR-activated nNOS activity and neuronal peroxynitrite levels by reducing nNOS phosphorylation at Ser(1412). The Ser(1412) phosphorylation is associated with increased nNOS activity. Supporting the notion that nNOS activity and peroxynitrite are deleterious following IR, inhibition of nNOS by its inhibitor 7-nitroindazole or reducing peroxynitrite by its scavenger FeTPPS decreased IR injury. GSNO also decreased the activation of AMP Kinase (AMPK) and its upstream kinase LKB1, both of which were activated in IR brain. AMPK has been implicated in nNOS activation via Ser(1412) phosphorylation. To determine whether AMPK activation is deleterious in the acute phase of IR, we treated animals after IR with AICAR (an AMPK activator) and compound c (an AMPK inhibitor). While AICAR potentiated, compound c reduced the IR injury.
Conclusions: Taken together, these results indicate an injurious nNOS/peroxynitrite/AMPK cycle following stroke, and GSNO treatment of IR inhibits this vicious cycle, resulting in neuroprotection and improved neurological function. GSNO is a natural component of the human body, and its exogenous administration to humans is not associated with any known side effects. Currently, the FDA-approved thrombolytic therapy suffers from a lack of neuronal protective activity. Because GSNO provides neuroprotection by ameliorating stroke's initial and causative injuries, it is a candidate of translational value for stroke therapy.
Figures
Similar articles
-
Targeting the nNOS/peroxynitrite/calpain system to confer neuroprotection and aid functional recovery in a mouse model of TBI.Brain Res. 2016 Jan 1;1630:159-70. doi: 10.1016/j.brainres.2015.11.015. Epub 2015 Nov 17. Brain Res. 2016. PMID: 26596859 Free PMC article.
-
Neuroprotection of S-nitrosoglutathione against ischemic injury by down-regulating Fas S-nitrosylation and downstream signaling.Neuroscience. 2013 Sep 17;248:290-8. doi: 10.1016/j.neuroscience.2013.06.012. Epub 2013 Jun 20. Neuroscience. 2013. PMID: 23792322
-
The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke.J Neurochem. 2012 Nov;123 Suppl 2(Suppl 2):86-97. doi: 10.1111/j.1471-4159.2012.07947.x. J Neurochem. 2012. PMID: 23050646 Free PMC article.
-
Investigation of S-Nitrosoglutathione in stroke: A systematic review and meta-analysis of literature in pre-clinical and clinical research.Exp Neurol. 2020 Jun;328:113262. doi: 10.1016/j.expneurol.2020.113262. Epub 2020 Feb 28. Exp Neurol. 2020. PMID: 32119935
-
Neuron protection as a therapeutic target in acute ischemic stroke.Curr Top Med Chem. 2009;9(14):1317-34. doi: 10.2174/156802609789869646. Curr Top Med Chem. 2009. PMID: 19849659 Review.
Cited by
-
Inhibition of the AMPK/nNOS pathway for neuroprotection in stroke.Neural Regen Res. 2016 Mar;11(3):398-9. doi: 10.4103/1673-5374.179039. Neural Regen Res. 2016. PMID: 27127467 Free PMC article. No abstract available.
-
S-nitrosylation of the Peroxiredoxin-2 promotes S-nitrosoglutathione-mediated lung cancer cells apoptosis via AMPK-SIRT1 pathway.Cell Death Dis. 2019 Apr 15;10(5):329. doi: 10.1038/s41419-019-1561-x. Cell Death Dis. 2019. PMID: 30988280 Free PMC article.
-
Amelioration of spinal cord injury in rats by blocking peroxynitrite/calpain activity.BMC Neurosci. 2018 Aug 13;19(1):50. doi: 10.1186/s12868-018-0450-z. BMC Neurosci. 2018. PMID: 30103682 Free PMC article.
-
Optogenetic Stimulation Reduces Neuronal Nitric Oxide Synthase Expression After Stroke.Transl Stroke Res. 2021 Apr;12(2):347-356. doi: 10.1007/s12975-020-00831-y. Epub 2020 Jul 13. Transl Stroke Res. 2021. PMID: 32661768 Free PMC article.
-
Role of neuronal nitric oxide synthase on cardiovascular functions in physiological and pathophysiological states.Nitric Oxide. 2020 Sep 1;102:52-73. doi: 10.1016/j.niox.2020.06.004. Epub 2020 Jun 23. Nitric Oxide. 2020. PMID: 32590118 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

