Differentiated effects of the multimodal antidepressant vortioxetine on sleep architecture: Part 2, pharmacological interactions in rodents suggest a role of serotonin-3 receptor antagonism
- PMID: 26174134
- PMCID: PMC4579402
- DOI: 10.1177/0269881115592347
Differentiated effects of the multimodal antidepressant vortioxetine on sleep architecture: Part 2, pharmacological interactions in rodents suggest a role of serotonin-3 receptor antagonism
Abstract
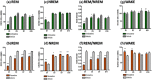
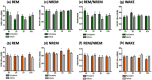
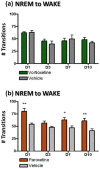
Antidepressants often disrupt sleep. Vortioxetine, a multimodal antidepressant acting through serotonin (5-HT) transporter (SERT) inhibition, 5-HT3, 5-HT7 and 5-HT1D receptor antagonism, 5-HT1B receptor partial agonism, and 5-HT1A receptor agonism, had fewer incidences of sleep-related adverse events reported in depressed patients. In the accompanying paper a polysomnographic electroencephalography (sleep-EEG) study of vortioxetine and paroxetine in healthy subjects indicated that at low/intermediate levels of SERT occupancy, vortioxetine affected rapid eye movement (REM) sleep differently than paroxetine. Here we investigated clinically meaningful doses (80-90% SERT occupancy) of vortioxetine and paroxetine on sleep-EEG in rats to further elucidate the serotoninergic receptor mechanisms mediating this difference. Cortical EEG, electromyography (EMG), and locomotion were recorded telemetrically for 10 days, following an acute dose, from rats receiving vortioxetine-infused chow or paroxetine-infused water and respective controls. Sleep stages were manually scored into active wake, quiet wake, and non-REM or REM sleep. Acute paroxetine or vortioxetine delayed REM onset latency (ROL) and decreased REM episodes. After repeated administration, vortioxetine yielded normal sleep-wake rhythms while paroxetine continued to suppress REM. Paroxetine, unlike vortioxetine, increased transitions from non-REM to wake, suggesting fragmented sleep. Next, we investigated the role of 5-HT3 receptors in eliciting these differences. The 5-HT3 receptor antagonist ondansetron significantly reduced paroxetine's acute effects on ROL, while the 5-HT3 receptor agonist SR57227A significantly increased vortioxetine's acute effect on ROL. Overall, our data are consistent with the clinical findings that vortioxetine impacts REM sleep differently than paroxetine, and suggests a role for 5-HT3 receptor antagonism in mitigating these differences.
Keywords: Sleep; antidepressant; serotonin; vortioxetine.
© The Author(s) 2015.
Conflict of interest statement
Figures

Similar articles
-
The rapid recovery of 5-HT cell firing induced by the antidepressant vortioxetine involves 5-HT(3) receptor antagonism.Int J Neuropsychopharmacol. 2013 Jun;16(5):1115-27. doi: 10.1017/S1461145712001058. Epub 2012 Oct 22. Int J Neuropsychopharmacol. 2013. PMID: 23089374
-
Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: Focus on glutamatergic and GABAergic neurotransmission.Neuropharmacology. 2016 Sep;108:73-81. doi: 10.1016/j.neuropharm.2016.04.023. Epub 2016 Apr 20. Neuropharmacology. 2016. PMID: 27106166
-
The multimodal antidepressant vortioxetine may facilitate pyramidal cell firing by inhibition of 5-HT3 receptor expressing interneurons: An in vitro study in rat hippocampus slices.Brain Res. 2018 Jun 15;1689:1-11. doi: 10.1016/j.brainres.2017.12.025. Epub 2017 Dec 21. Brain Res. 2018. PMID: 29274875
-
Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data.Pharmacol Ther. 2015 Jan;145:43-57. doi: 10.1016/j.pharmthera.2014.07.001. Epub 2014 Jul 9. Pharmacol Ther. 2015. PMID: 25016186 Review.
-
Modes and nodes explain the mechanism of action of vortioxetine, a multimodal agent (MMA): blocking 5HT3 receptors enhances release of serotonin, norepinephrine, and acetylcholine.CNS Spectr. 2015 Oct;20(5):455-9. doi: 10.1017/S1092852915000346. Epub 2015 Jun 30. CNS Spectr. 2015. PMID: 26122791 Review.
Cited by
-
Effects of Vortioxetine on Sleep Architecture of Adolescents with Major Depressive Disorder.Clocks Sleep. 2023 Oct 24;5(4):627-638. doi: 10.3390/clockssleep5040042. Clocks Sleep. 2023. PMID: 37987393 Free PMC article.
-
Gut microbial β-glucuronidases influence endobiotic homeostasis and are modulated by diverse therapeutics.Cell Host Microbe. 2024 Jun 12;32(6):925-944.e10. doi: 10.1016/j.chom.2024.04.018. Epub 2024 May 15. Cell Host Microbe. 2024. PMID: 38754417 Free PMC article.
-
Phytoconstituents Targeting the Serotonin 5-HT3 Receptor: Promising Therapeutic Strategies for Neurological Disorders.ACS Pharmacol Transl Sci. 2024 Jun 4;7(6):1694-1710. doi: 10.1021/acsptsci.4c00084. eCollection 2024 Jun 14. ACS Pharmacol Transl Sci. 2024. PMID: 38898946 Free PMC article. Review.
-
Effectiveness of Vortioxetine for the Treatment of Emotional Blunting in Patients with Major Depressive Disorder Experiencing Inadequate Response to SSRI/SNRI Monotherapy in Spain: Results from the COMPLETE Study.Neuropsychiatr Dis Treat. 2024 Jul 30;20:1475-1489. doi: 10.2147/NDT.S473056. eCollection 2024. Neuropsychiatr Dis Treat. 2024. PMID: 39100571 Free PMC article.
-
Preliminary evidence that vortioxetine may improve sleep quality in depressed patients with insomnia: a retrospective questionnaire analysis.Br J Clin Pharmacol. 2019 Jan;85(1):240-244. doi: 10.1111/bcp.13772. Epub 2018 Oct 24. Br J Clin Pharmacol. 2019. PMID: 30328132 Free PMC article.
References
-
- Adrien J, Tissier MH, Lanfumey L, et al. (1992) Central action of 5-HT3 receptor ligands in the regulation of sleep-wakefulness and raphe neuronal activity in the rat. Neuropharmacology 31: 519–529. - PubMed
-
- Aloe F, de Azevedo AP, Hasan R. (2005) Sleep-wake cycle mechanisms. Rev Bras Psiquiatr 27: 33–39. - PubMed
-
- Amici R, Sanford LD, Kearney K, et al. (2004) A serotonergic (5-HT2) receptor mechanism in the laterodorsal tegmental nucleus participates in regulating the pattern of rapid-eye-movement sleep occurrence in the rat. Brain Res 996: 9–18. - PubMed
-
- Areberg J, Luntang-Jensen M, Sogaard B, et al. (2012) Occupancy of the serotonin transporter after administration of Lu AA21004 and its relation to plasma concentration in healthy subjects. Basic Clin Pharmacol Toxicol 110: 401–404. - PubMed
-
- Arfken CL, Joseph A, Sandhu GR, et al. (2014) The status of sleep abnormalities as a diagnostic test for major depressive disorder. J Affect Disord 156: 36–45. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

