Prediction of outcomes by early treatment responses in childhood T-cell acute lymphoblastic leukemia: a retrospective study in China
- PMID: 26174476
- PMCID: PMC4502910
- DOI: 10.1186/s12887-015-0390-z
Prediction of outcomes by early treatment responses in childhood T-cell acute lymphoblastic leukemia: a retrospective study in China
Abstract
Background: Early treatment responses are important prognostic factors in childhood T-cell acute lymphoblastic leukemia (T-ALL) patients. The predictive values of early treatment responses in Chinese childhood T-ALL patients were still unknown.
Methods: From January 2003 to December 2012, 74 consecutive patients aged ≤ 15 years with newly diagnosed T-ALL were treated with BCH-2003 protocol or CCLG-2008 protocol in the Department of Pediatric, Institute of Hematology and Blood Diseases Hospital in China. Predictive values of early treatment responses, including prednisone response, bone marrow morphology at day 15 and day 33 during induction chemotherapy, and minimal residual disease (MRD) monitored by flow cytometry after induction therapy (time point 1, TP1) and before consolidation therapy (time point 2, TP2), were analyzed.
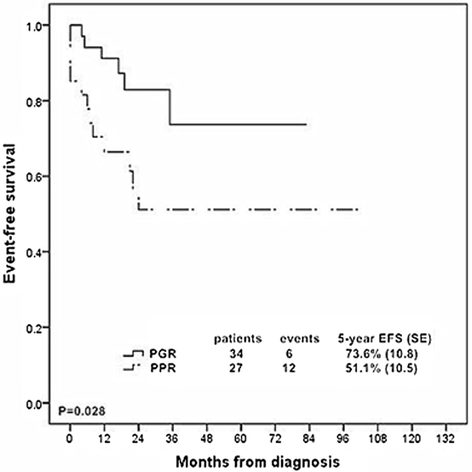
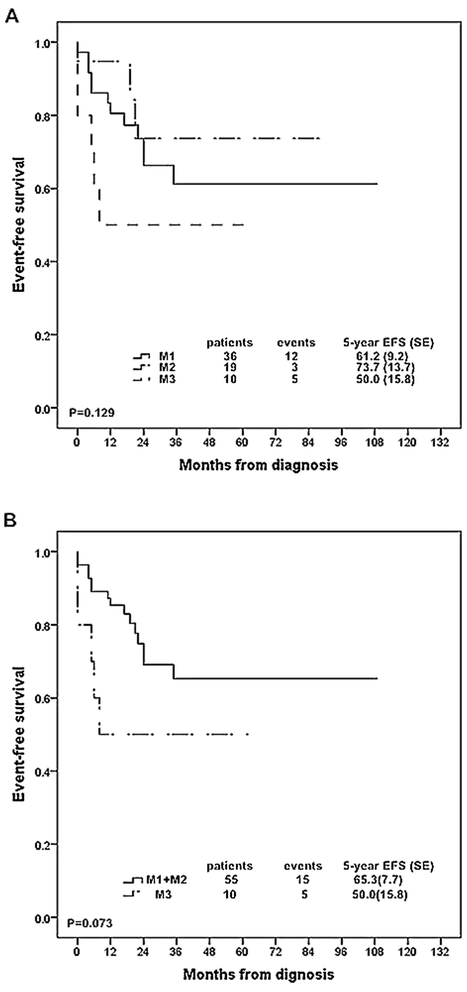
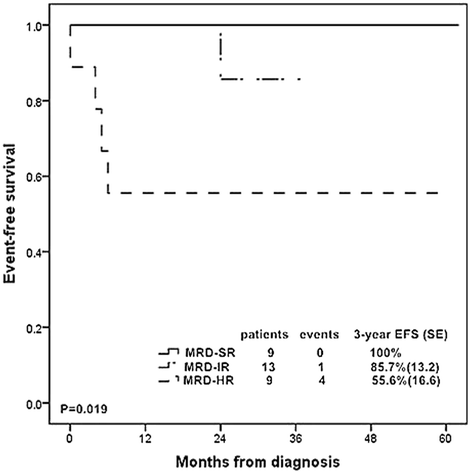
Results: The 5-year event free survival (EFS) and overall survival (OS) rates for these patients were 62.5% (SE, 6.4) and 62.7% (SE, 6.6), respectively. Prednisone poor responder was strongly associated with increased chance of induction failure (14.8%) and decreased survival rate (5 year EFS rate, 51.1 % (SE, 10.5)). Patients with ≥ 25% blast cells in bone marrow at day 15 were more likely to have an inferior outcome. 93.2% of the T-ALL patients achieved complete remission at day 33 while patients with resistant disease all died of disease progression. MRD ≥ 10(-2) at TP1 or MRD ≥ 10(-3) at TP2 was significantly related to dismal prognosis. Risk groups classified by MRD at two time points could stratify patients into different groups: 29.0% of the patients were MRD standard risk (MRD < 10(-4) at both time points) with 3-year EFS rate of 100%, 29.0% were MRD high risk (MRD ≥ 10(-2) at TP1 or MRD ≥ 10(-2) at TP2) with 3-year EFS rate of 55.6% (SE, 16.6) , and the rest of patients were defined as MRD intermediate risk with 3-year EFS rate of 85.7% (SE, 13.2).
Conclusion: Our study demonstrated that MRD was the most powerful predictor of treatment outcome in childhood T-ALL patients and conventional morphological assessments of treatment response still played important roles in predicting treatment outcome and tailoring treatment intensity especially in countries with inadequate skills or financial resources for MRD monitoring.
Figures

Similar articles
-
[Treatment outcome of childhood standard-risk and median-risk acute lymphoblastic leukemia with CCLG-2008 protocol].Zhonghua Er Ke Za Zhi. 2014 Jun;52(6):449-54. Zhonghua Er Ke Za Zhi. 2014. PMID: 25190166 Chinese.
-
[Monitoring of minimal residual disease in children with acute lymphoblastic leukemia and its prognostic significance].Zhonghua Er Ke Za Zhi. 2010 Mar;48(3):180-4. Zhonghua Er Ke Za Zhi. 2010. PMID: 20426951 Chinese.
-
Clinical features, early treatment responses, and outcomes of pediatric acute lymphoblastic leukemia in China with or without specific fusion transcripts: a single institutional study of 1,004 patients.Am J Hematol. 2012 Nov;87(11):1022-7. doi: 10.1002/ajh.23307. Epub 2012 Aug 22. Am J Hematol. 2012. PMID: 22911440
-
Minimal Residual Disease Evaluation in Childhood Acute Lymphoblastic Leukemia: A Clinical Evidence Review.Ont Health Technol Assess Ser. 2016 Mar 8;16(7):1-52. eCollection 2016. Ont Health Technol Assess Ser. 2016. PMID: 27099643 Free PMC article. Review.
-
Clinical importance of speed of response to therapy in childhood lymphoblastic leukaemia.Leuk Lymphoma. 1998 Nov;31(5-6):501-6. doi: 10.3109/10428199809057609. Leuk Lymphoma. 1998. PMID: 9922040 Review.
Cited by
-
miR-103 inhibits proliferation and sensitizes hemopoietic tumor cells for glucocorticoid-induced apoptosis.Oncotarget. 2017 Jan 3;8(1):472-489. doi: 10.18632/oncotarget.13447. Oncotarget. 2017. PMID: 27888798 Free PMC article.
-
Early T-cell precursor acute lymphoblastic leukemia and other subtypes: a retrospective case report from a single pediatric center in China.J Cancer Res Clin Oncol. 2021 Sep;147(9):2775-2788. doi: 10.1007/s00432-021-03551-4. Epub 2021 Mar 2. J Cancer Res Clin Oncol. 2021. PMID: 33651142 Free PMC article.
-
Prognostic Significance of Mixed-Lineage Leukemia (MLL) Gene Detected by Real-Time Fluorescence Quantitative PCR Assay in Acute Myeloid Leukemia.Med Sci Monit. 2016 Aug 26;22:3009-17. doi: 10.12659/msm.900429. Med Sci Monit. 2016. PMID: 27561414 Free PMC article.
-
Integrated genomic analyses identify high-risk factors and actionable targets in T-cell acute lymphoblastic leukemia.Blood Sci. 2022 Feb 4;4(1):16-28. doi: 10.1097/BS9.0000000000000102. eCollection 2022 Jan. Blood Sci. 2022. PMID: 35399540 Free PMC article.
-
Survival and Treatment Outcomes of Childhood Acute Lymphoblastic Leukemia in a Low-Middle Income Country: A Single-Center Experience in West Java, Indonesia.J Blood Med. 2024 Feb 19;15:77-85. doi: 10.2147/JBM.S438042. eCollection 2024. J Blood Med. 2024. PMID: 38405084 Free PMC article.
References
-
- Uckun FM, Sensel MG, Sun L, Steinherz PG, Trigg ME, Heerema NA, Sather HN, Reaman GH, Gaynon PS. Biology and treatment of childhood T-lineage acute lymphoblastic leukemia. Blood. 1998;91(3):735–746. - PubMed
-
- Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, Cohen HJ, Sallan SE, Asselin BL. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21(19):3616–3622. doi: 10.1200/JCO.2003.10.116. - DOI - PubMed
-
- Möricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dördelmann M, Löning L, Beier R, Ludwig WD, Ratei R, Harbott J, Boos J, Mann G, Niggli F, Feldges A, Henze G, Welte K, Beck JD, Klingebiel T, Niemeyer C, Zintl F, Bode U, Urban C, Wehinger H, Niethammer D, Riehm H, Schrappe M, German-Austrian-Swiss ALL-BFM Study Group Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111(9):4477–4489. doi: 10.1182/blood-2007-09-112920. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

