Acid-sensing ion channel 1a drives AMPA receptor plasticity following ischaemia and acidosis in hippocampal CA1 neurons
- PMID: 26174503
- PMCID: PMC4594240
- DOI: 10.1113/JP270701
Acid-sensing ion channel 1a drives AMPA receptor plasticity following ischaemia and acidosis in hippocampal CA1 neurons
Abstract
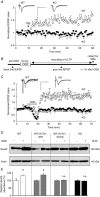
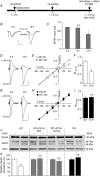
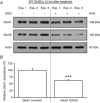
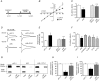
Key points: The hippocampal CA1 region is highly vulnerable to ischaemic stroke. Two forms of AMPA receptor (AMPAR) plasticity - an anoxic form of long-term potentiation and a delayed increase in Ca(2+) -permeable (CP) AMPARs - contribute to this susceptibility by increasing excitotoxicity. In CA1, the acid-sensing ion channel 1a (ASIC1a) is known to facilitate LTP and contribute to ischaemic acidotoxicity. We have examined the role of ASIC1a in AMPAR ischaemic plasticity in organotypic hippocampal slice cultures exposed to oxygen glucose deprivation (a model of ischaemic stroke), and in hippocampal pyramidal neuron cultures exposed to acidosis. We find that ASIC1a activation promotes both forms of AMPAR plasticity and that neuroprotection, by inhibiting ASIC1a, circumvents any further benefit of blocking CP-AMPARs. Our observations establish a new interaction between acidotoxicity and excitotoxicity, and provide insight into the role of ASIC1a and CP-AMPARs in neurodegeneration. Specifically, we propose that ASIC1a activation drives certain post-ischaemic forms of CP-AMPAR plasticity.
Abstract: The CA1 region of the hippocampus is particularly vulnerable to ischaemic damage. While NMDA receptors play a major role in excitotoxicity, it is thought to be exacerbated in this region by two forms of post-ischaemic AMPA receptor (AMPAR) plasticity - namely, anoxic long-term potentiation (a-LTP), and a delayed increase in the prevalence of Ca(2+) -permeable GluA2-lacking AMPARs (CP-AMPARs). The acid-sensing ion channel 1a (ASIC1a), which is expressed in CA1 pyramidal neurons, is also known to contribute to post-ischaemic neuronal death and to physiologically induced LTP. This raises the question does ASIC1a activation drive the post-ischaemic forms of AMPAR plasticity in CA1 pyramidal neurons? We have tested this by examining organotypic hippocampal slice cultures (OHSCs) exposed to oxygen glucose deprivation (OGD), and dissociated cultures of hippocampal pyramidal neurons (HPNs) exposed to low pH (acidosis). We find that both a-LTP and the delayed increase in the prevalence of CP-AMPARs are dependent on ASIC1a activation during ischaemia. Indeed, acidosis alone is sufficient to induce the increase in CP-AMPARs. We also find that inhibition of ASIC1a channels circumvents any potential neuroprotective benefit arising from block of CP-AMPARs. By demonstrating that ASIC1a activation contributes to post-ischaemic AMPAR plasticity, our results identify a functional interaction between acidotoxicity and excitotoxicity in hippocampal CA1 cells, and provide insight into the role of ASIC1a and CP-AMPARs as potential drug targets for neuroprotection. We thus propose that ASIC1a activation can drive certain forms of CP-AMPAR plasticity, and that inhibiting ASIC1a affords neuroprotection.
© 2015 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures
Comment in
-
Acid-sensing ion channel 1a induces AMPA receptor plasticity: a link between acidotoxicity and excitotoxicity in hippocampal CA1 neurons.J Physiol. 2016 Feb 15;594(4):803-5. doi: 10.1113/JP271814. J Physiol. 2016. PMID: 26876443 Free PMC article. No abstract available.
Similar articles
-
Acid-sensing ion channel 1a induces AMPA receptor plasticity: a link between acidotoxicity and excitotoxicity in hippocampal CA1 neurons.J Physiol. 2016 Feb 15;594(4):803-5. doi: 10.1113/JP271814. J Physiol. 2016. PMID: 26876443 Free PMC article. No abstract available.
-
Ischemia-induced synaptic plasticity drives sustained expression of calcium-permeable AMPA receptors in the hippocampus.Neuropharmacology. 2013 Feb;65:114-22. doi: 10.1016/j.neuropharm.2012.09.016. Epub 2012 Oct 4. Neuropharmacology. 2013. PMID: 23041538
-
PARP-1 activation causes neuronal death in the hippocampal CA1 region by increasing the expression of Ca(2+)-permeable AMPA receptors.Neurobiol Dis. 2014 Oct;70:43-52. doi: 10.1016/j.nbd.2014.05.023. Epub 2014 Jun 20. Neurobiol Dis. 2014. PMID: 24954469
-
The AMPAR subunit GluR2: still front and center-stage.Brain Res. 2000 Dec 15;886(1-2):190-207. doi: 10.1016/s0006-8993(00)02951-6. Brain Res. 2000. PMID: 11119696 Review.
-
Role of ASIC1a in Normal and Pathological Synaptic Plasticity.Rev Physiol Biochem Pharmacol. 2020;177:83-100. doi: 10.1007/112_2020_45. Rev Physiol Biochem Pharmacol. 2020. PMID: 32789788 Review.
Cited by
-
Acute inhibition of acid sensing ion channel 1a after spinal cord injury selectively affects excitatory synaptic transmission, but not intrinsic membrane properties, in deep dorsal horn interneurons.PLoS One. 2023 Nov 8;18(11):e0289053. doi: 10.1371/journal.pone.0289053. eCollection 2023. PLoS One. 2023. PMID: 37939057 Free PMC article.
-
Probing conformational changes during activation of ASIC1a by an optical tweezer and by methanethiosulfonate-based cross-linkers.PLoS One. 2022 Jul 8;17(7):e0270762. doi: 10.1371/journal.pone.0270762. eCollection 2022. PLoS One. 2022. PMID: 35802631 Free PMC article.
-
ASIC1a regulates insular long-term depression and is required for the extinction of conditioned taste aversion.Nat Commun. 2016 Dec 7;7:13770. doi: 10.1038/ncomms13770. Nat Commun. 2016. PMID: 27924869 Free PMC article.
-
Ca2+ -permeable AMPA receptors and their auxiliary subunits in synaptic plasticity and disease.J Physiol. 2021 May;599(10):2655-2671. doi: 10.1113/JP279029. Epub 2021 Feb 21. J Physiol. 2021. PMID: 33533533 Free PMC article.
-
Acid-Sensing Ion Channels as Potential Therapeutic Targets in Neurodegeneration and Neuroinflammation.Mediators Inflamm. 2017;2017:3728096. doi: 10.1155/2017/3728096. Epub 2017 Sep 19. Mediators Inflamm. 2017. PMID: 29056828 Free PMC article. Review.
References
-
- Ahlgren H, Henjum K, Ottersen OP & Runden‐Pran E (2011). Validation of organotypical hippocampal slice cultures as an ex vivo model of brain ischemia: different roles of NMDA receptors in cell death signalling after exposure to NMDA or oxygen and glucose deprivation. Cell Tissue Res 345, 329–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

