Development and functions of the choroid plexus-cerebrospinal fluid system
- PMID: 26174708
- PMCID: PMC4629451
- DOI: 10.1038/nrn3921
Development and functions of the choroid plexus-cerebrospinal fluid system
Abstract
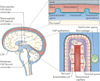
The choroid plexus (ChP) is the principal source of cerebrospinal fluid (CSF), which has accepted roles as a fluid cushion and a sink for nervous system waste in vertebrates. Various animal models have provided insights into how the ChP-CSF system develops and matures. In addition, recent studies have uncovered new, active roles for this dynamic system in the regulation of neural stem cells, critical periods and the overall health of the nervous system. Together, these findings have brought about a paradigm shift in our understanding of brain development and health, and have stimulated new initiatives for the treatment of neurological disease.
Figures



References
-
- Damkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev. 2013;93:1847–1892. - PubMed
-
- Koh L, et al. Development of cerebrospinal fluid absorption sites in the pig and rat: connections between the subarachnoid space and lymphatic vessels in the olfactory turbinates. Anat Embryol (Berl) 2006;211:335–344. - PubMed
-
- Johnston M, Zakharov A, Koh L, Armstrong D. Subarachnoid injection of Microfil reveals connections between cerebrospinal fluid and nasal lymphatics in the non-human primate. Neuropathol Appl Neurobiol. 2005;31:632–640. - PubMed
-
- Mollanji R, Bozanovic-Sosic R, Zakharov A, Makarian L, Johnston MG. Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1593–R1599. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

