Canine mammary cancer cells direct macrophages toward an intermediate activation state between M1/M2
- PMID: 26174804
- PMCID: PMC4502937
- DOI: 10.1186/s12917-015-0473-y
Canine mammary cancer cells direct macrophages toward an intermediate activation state between M1/M2
Abstract
Background: Canine mammary carcinoma is the most common cancer in female dogs and is often fatal due to the development of distance metastasis. The microenvironment of a tumour often contains abundant infiltrates of macrophages called tumour-associated macrophages (TAMs). TAMs express an activated phenotype, termed M2, which sustains proliferation of cancer cells, and has been correlated with poor clinical outcomes in human cancer patients. Cancer cells themselves have been implicated in stimulating the conversion of macrophages to a TAM with an M2 phenotype. This process has yet to be fully elucidated. Here we investigate the interplay between cancer cells and macrophages in the context of canine mammary carcinoma.
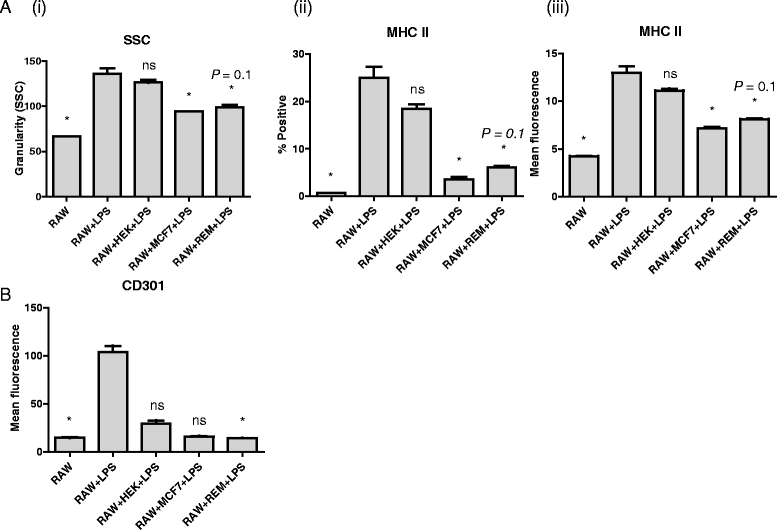
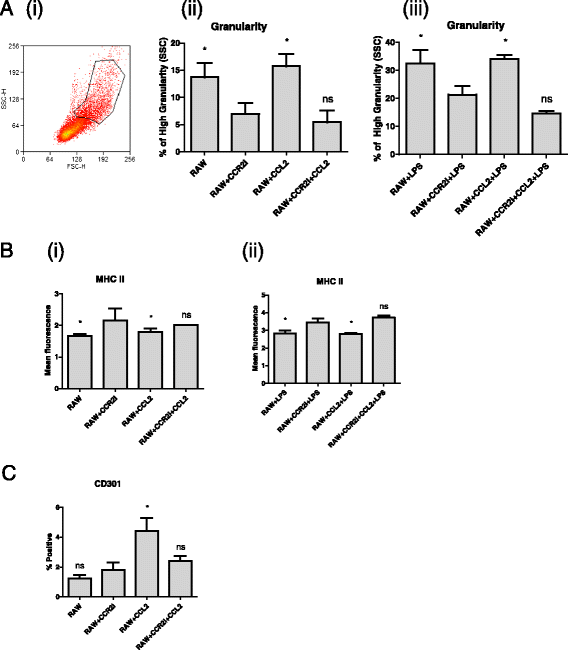
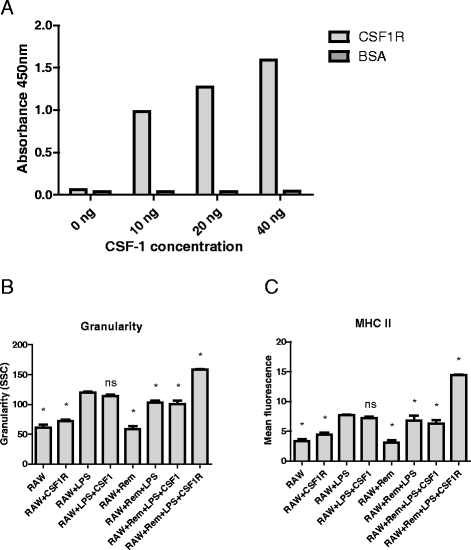
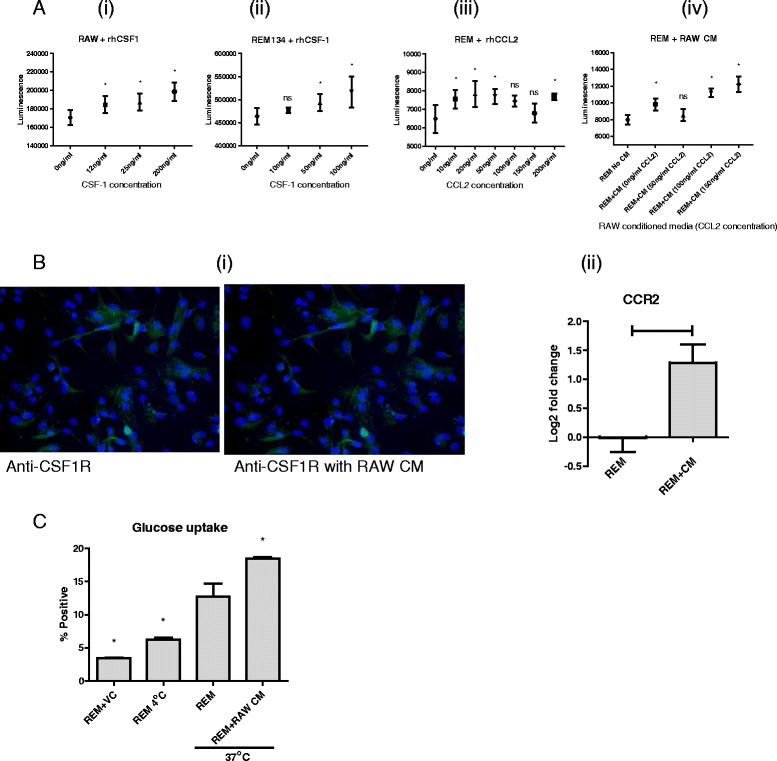
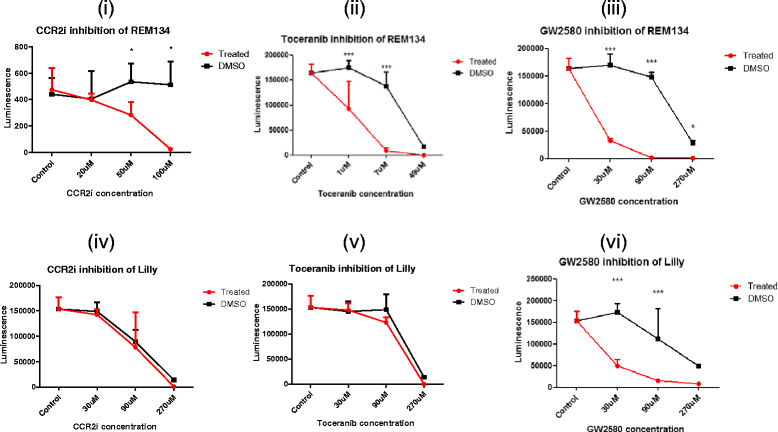
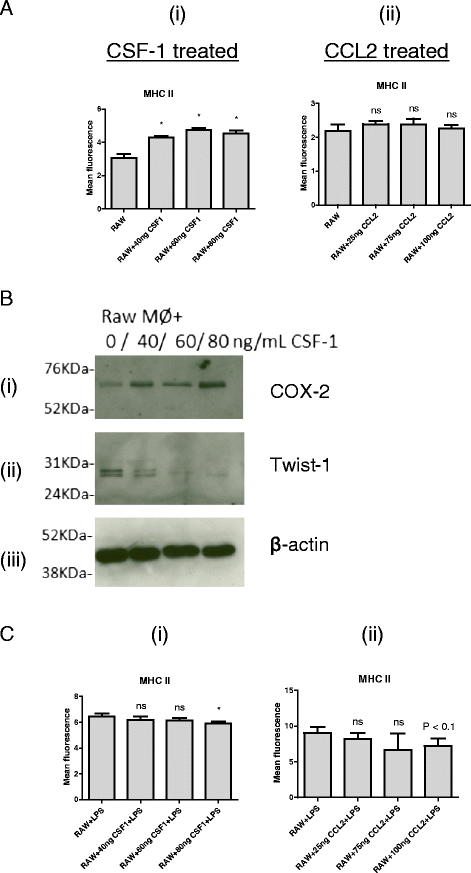
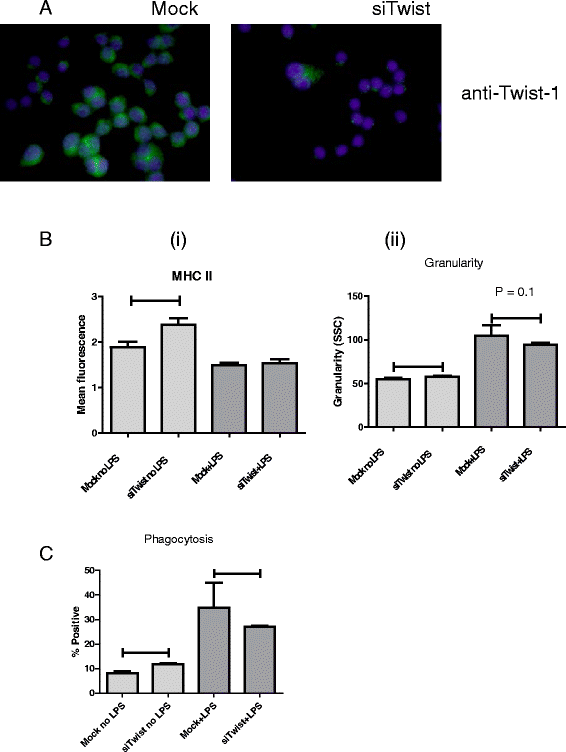
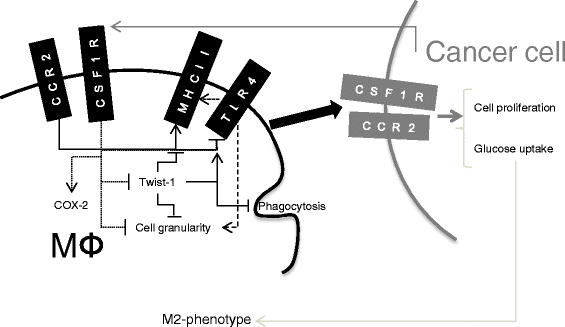
Results: We show that cancer cells inhibit lipopolysaccharide (LPS)-induced macrophage activation. Further, we show that macrophage associated proteins, colony-stimulating factor (CSF)-1 and C-C motif ligand (CCL)-2, stimulate macrophages and are responsible for the effects of cancer cells on macrophages. We suggest the existence of a feedback loop between macrophages and cancer cells; while cancer cells influence the phenotype of the TAMs through CSF-1 and CCL2, the macrophages induce canine mammary cancer cells to upregulate their own expression of the receptors for CSF-1 and CCL2 and increase the cancer cellular metabolic activity. However, these cytokines in isolation induce a phenotypic state in macrophages that is between M1 and M2 phenotypes.
Conclusions: Overall, our results demonstrate the extent to which canine mammary carcinoma cells influence the macrophage phenotype and the relevance of a feedback loop between these cells, involving CSF-1 and CCL2 as important mediators.
Figures
Similar articles
-
Tumour-associated macrophages: Relation with progression and invasiveness, and assessment of M1/M2 macrophages in canine mammary tumours.Vet J. 2018 Apr;234:119-125. doi: 10.1016/j.tvjl.2018.02.016. Epub 2018 Feb 27. Vet J. 2018. PMID: 29680383
-
Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization.BMC Cancer. 2013 Sep 17;13:421. doi: 10.1186/1471-2407-13-421. BMC Cancer. 2013. PMID: 24044575 Free PMC article.
-
Diverse in vivo effects of soluble and membrane-bound M-CSF on tumor-associated macrophages in lymphoma xenograft model.Oncotarget. 2016 Jan 12;7(2):1354-66. doi: 10.18632/oncotarget.6362. Oncotarget. 2016. PMID: 26595525 Free PMC article.
-
CSF-1R signaling in health and disease: a focus on the mammary gland.J Mammary Gland Biol Neoplasia. 2014 Jul;19(2):149-59. doi: 10.1007/s10911-014-9320-1. Epub 2014 Jun 10. J Mammary Gland Biol Neoplasia. 2014. PMID: 24912655 Review.
-
Macrophage phenotype-switching in cancer.Eur J Pharmacol. 2022 Sep 15;931:175229. doi: 10.1016/j.ejphar.2022.175229. Epub 2022 Aug 21. Eur J Pharmacol. 2022. PMID: 36002039 Review.
Cited by
-
Immune Cells and Immunoglobulin Expression in the Mammary Gland Tumors of Dog.Animals (Basel). 2021 Apr 21;11(5):1189. doi: 10.3390/ani11051189. Animals (Basel). 2021. PMID: 33919282 Free PMC article.
-
Bidirectional Regulation of COX-2 Expression Between Cancer Cells and Macrophages.Anticancer Res. 2018 May;38(5):2811-2817. doi: 10.21873/anticanres.12525. Anticancer Res. 2018. PMID: 29715103 Free PMC article.
-
Deciphering the performance of macrophages in tumour microenvironment: a call for precision immunotherapy.J Hematol Oncol. 2024 Jun 11;17(1):44. doi: 10.1186/s13045-024-01559-0. J Hematol Oncol. 2024. PMID: 38863020 Free PMC article. Review.
-
A blocking antibody against canine CSF-1R maturated by limited CDR mutagenesis.Antib Ther. 2020 Aug 12;3(3):193-204. doi: 10.1093/abt/tbaa018. eCollection 2020 Jul. Antib Ther. 2020. PMID: 33937625 Free PMC article.
-
Metabolic Alterations in Canine Mammary Tumors.Animals (Basel). 2023 Aug 30;13(17):2757. doi: 10.3390/ani13172757. Animals (Basel). 2023. PMID: 37685021 Free PMC article. Review.
References
-
- Aharinejad S, Paulus P, Sioud M, Hofmann M, Zins K, Schäfer R, et al. Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res. 2004;64:5378–5384. doi: 10.1158/0008-5472.CAN-04-0961. - DOI - PubMed
-
- Biswas SK, Allavena P, Mantovani A: Tumor-associated macrophages: functional diversity, clinical significance, and open questions. In Seminars in immunopathology. Volume 35. Springer; 2013:585–600. - PubMed
-
- Chambers SK, Kacinski BM, Ivins CM, Carcangiu ML. Overexpression of epithelial macrophage colony-stimulating factor (CSF-1) and CSF-1 receptor: a poor prognostic factor in epithelial ovarian cancer, contrasted with a protective effect of stromal CSF-1. Clin Cancer Res. 1997;3:999–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

