Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model
- PMID: 26174883
- PMCID: PMC4696021
- DOI: 10.1002/ijc.29686
Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model
Abstract
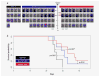
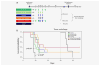
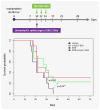
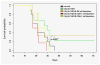
Glioblastoma is the most aggressive primary central nervous system malignancy with a poor prognosis in patients. Despite the need for better treatments against glioblastoma, very little progress has been made in discovering new therapies that exhibit superior survival benefit than the standard of care. Immunotherapy has been shown to be a promising treatment modality that could help improve clinical outcomes of glioblastoma patients by assisting the immune system to overcome the immunosuppressive tumor environment. Interleukin-15 (IL-15), a cytokine shown to activate several effector components of the immune system, may serve as an excellent immunotherapeutic candidate for the treatment of glioblastoma. Thus, we evaluated the efficacy of an IL-15 superagonist complex (IL-15N72D:IL-15RαSu-Fc; also known as ALT-803) in a murine GL261-luc glioblastoma model. We show that ALT-803, as a single treatment as well as in combination with anti-PD-1 antibody or stereotactic radiosurgery, exhibits a robust antitumor immune response resulting in a prolonged survival including complete remission in tumor bearing mice. In addition, ALT-803 treatment results in long-term immune memory against glioblastoma tumor rechallenge. Flow cytometric analysis of tumor infiltrating immune cells shows that ALT-803 leads to increased percentage of CD8+-cell infiltration, but not the NK cells, and IFN-γ production into the tumor microenvironment. Cell depletion studies, in accordance with the flow cytometric results, show that the ALT-803 therapeutic effect is dependent on CD4+ and CD8+ cells. These results provide a rationale for evaluating the therapeutic activity of ALT-803 against glioblastoma in the clinical setting.
Keywords: ALT-803; IL-15 superagonist; anti-PD-1; glioblastoma.
© 2015 UICC.
Conflict of interest statement
Conflict of Interest: Hing C. Wong, Peter R. Rhode, Warren D. Marcus, Sarah Alter, and Emily K. Jeng are the employees and stockholders of Altor Bioscience Corporation.
Drew M. Pardoll: Research Funding: Bristol Myer Squibb
Michael Lim: Research Funding: Bristol Myer Squibb, Celldex Therapeutics, Immunocellular Therapeutics Ltd, Altor BioScience Corporation, Agenus, Merck, and Accuray.
Figures
Similar articles
-
IL-15 superagonist/IL-15RαSushi-Fc fusion complex (IL-15SA/IL-15RαSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas.Oncotarget. 2016 Mar 29;7(13):16130-45. doi: 10.18632/oncotarget.7470. Oncotarget. 2016. PMID: 26910920 Free PMC article.
-
New interleukin-15 superagonist (IL-15SA) significantly enhances graft-versus-tumor activity.Oncotarget. 2017 Jul 4;8(27):44366-44378. doi: 10.18632/oncotarget.17875. Oncotarget. 2017. PMID: 28574833 Free PMC article.
-
Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model.Cancer Immunol Res. 2016 Feb;4(2):124-35. doi: 10.1158/2326-6066.CIR-15-0151. Epub 2015 Nov 6. Cancer Immunol Res. 2016. PMID: 26546453
-
Immunotherapy for the treatment of breast cancer: checkpoint blockade, cancer vaccines, and future directions in combination immunotherapy.Clin Adv Hematol Oncol. 2016 Nov;14(11):922-933. Clin Adv Hematol Oncol. 2016. PMID: 27930644 Review.
-
Immune Checkpoint Modulators: An Emerging Antiglioma Armamentarium.J Immunol Res. 2016;2016:4683607. doi: 10.1155/2016/4683607. Epub 2016 Jan 4. J Immunol Res. 2016. PMID: 26881264 Free PMC article. Review.
Cited by
-
Regulatory T cells (Tregs): A major immune checkpoint to consider in combinatorial therapeutic HIV-1 vaccines.Hum Vaccin Immunother. 2018 Jun 3;14(6):1432-1437. doi: 10.1080/21645515.2018.1434384. Epub 2018 Feb 23. Hum Vaccin Immunother. 2018. PMID: 29381418 Free PMC article. Review.
-
Mechanisms involved in IL-15 superagonist enhancement of anti-PD-L1 therapy.J Immunother Cancer. 2019 Mar 21;7(1):82. doi: 10.1186/s40425-019-0551-y. J Immunother Cancer. 2019. PMID: 30898149 Free PMC article.
-
Immune microenvironment of experimental rat C6 gliomas resembles human glioblastomas.Sci Rep. 2017 Dec 14;7(1):17556. doi: 10.1038/s41598-017-17752-w. Sci Rep. 2017. PMID: 29242629 Free PMC article.
-
Next-Generation Immunotherapies to Improve Anticancer Immunity.Front Pharmacol. 2021 Jan 11;11:566401. doi: 10.3389/fphar.2020.566401. eCollection 2020. Front Pharmacol. 2021. PMID: 33505304 Free PMC article. Review.
-
Therapy of Established Tumors with Rationally Designed Multiple Agents Targeting Diverse Immune-Tumor Interactions: Engage, Expand, Enable.Cancer Immunol Res. 2021 Feb;9(2):239-252. doi: 10.1158/2326-6066.CIR-20-0638. Epub 2020 Dec 22. Cancer Immunol Res. 2021. PMID: 33355290 Free PMC article.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine. 2005;352:987–96. - PubMed
-
- Authier A, Farrand KJ, Broadley KW, et al. Enhanced immunosuppression by therapy-exposed glioblastoma multiforme tumor cells. International Journal of Cancer. 2015;136:2566–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

