Accurate and representative decoding of the neural drive to muscles in humans with multi-channel intramuscular thin-film electrodes
- PMID: 26174910
- PMCID: PMC4575568
- DOI: 10.1113/JP270902
Accurate and representative decoding of the neural drive to muscles in humans with multi-channel intramuscular thin-film electrodes
Abstract
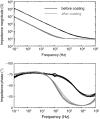
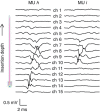
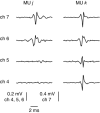
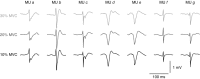
Intramuscular electrodes developed over the past 80 years can record the concurrent activity of only a few motor units active during a muscle contraction. We designed, produced and tested a novel multi-channel intramuscular wire electrode that allows in vivo concurrent recordings of a substantially greater number of motor units than with conventional methods. The electrode has been extensively tested in deep and superficial human muscles. The performed tests indicate the applicability of the proposed technology in a variety of conditions. The electrode represents an important novel technology that opens new avenues in the study of the neural control of muscles in humans. We describe the design, fabrication and testing of a novel multi-channel thin-film electrode for detection of the output of motoneurones in vivo and in humans, through muscle signals. The structure includes a linear array of 16 detection sites that can sample intramuscular electromyographic activity from the entire muscle cross-section. The structure was tested in two superficial muscles (the abductor digiti minimi (ADM) and the tibialis anterior (TA)) and a deep muscle (the genioglossus (GG)) during contractions at various forces. Moreover, surface electromyogram (EMG) signals were concurrently detected from the TA muscle with a grid of 64 electrodes. Surface and intramuscular signals were decomposed into the constituent motor unit (MU) action potential trains. With the intramuscular electrode, up to 31 MUs were identified from the ADM muscle during an isometric contraction at 15% of the maximal force (MVC) and 50 MUs were identified for a 30% MVC contraction of TA. The new electrode detects different sources from a surface EMG system, as only one MU spike train was found to be common in the decomposition of the intramuscular and surface signals acquired from the TA. The system also allowed access to the GG muscle, which cannot be analysed with surface EMG, with successful identification of MU activity. With respect to classic detection systems, the presented thin-film structure enables recording from large populations of active MUs of deep and superficial muscles and thus can provide a faithful representation of the neural drive sent to a muscle.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures







Similar articles
-
Multi-channel intramuscular and surface EMG decomposition by convolutive blind source separation.J Neural Eng. 2016 Apr;13(2):026027. doi: 10.1088/1741-2560/13/2/026027. Epub 2016 Feb 29. J Neural Eng. 2016. PMID: 26924829
-
Experimental analysis of accuracy in the identification of motor unit spike trains from high-density surface EMG.IEEE Trans Neural Syst Rehabil Eng. 2010 Jun;18(3):221-9. doi: 10.1109/TNSRE.2010.2041593. Epub 2010 Feb 8. IEEE Trans Neural Syst Rehabil Eng. 2010. PMID: 20144921
-
Estimating reflex responses in large populations of motor units by decomposition of the high-density surface electromyogram.J Physiol. 2015 Oct 1;593(19):4305-18. doi: 10.1113/JP270635. Epub 2015 Aug 2. J Physiol. 2015. PMID: 26115007 Free PMC article.
-
Decoding the neural drive to muscles from the surface electromyogram.Clin Neurophysiol. 2010 Oct;121(10):1616-23. doi: 10.1016/j.clinph.2009.10.040. Epub 2010 May 4. Clin Neurophysiol. 2010. PMID: 20444646 Review.
-
NeuroMechanics: Electrophysiological and computational methods to accurately estimate the neural drive to muscles in humans in vivo.J Electromyogr Kinesiol. 2024 Jun;76:102873. doi: 10.1016/j.jelekin.2024.102873. Epub 2024 Mar 7. J Electromyogr Kinesiol. 2024. PMID: 38518426 Review.
Cited by
-
The reliability of methods to estimate the number and size of human motor units and their use with large limb muscles.Eur J Appl Physiol. 2018 Apr;118(4):767-775. doi: 10.1007/s00421-018-3811-5. Epub 2018 Jan 22. Eur J Appl Physiol. 2018. PMID: 29356950 Free PMC article.
-
A motor unit-based model of muscle fatigue.PLoS Comput Biol. 2017 Jun 2;13(6):e1005581. doi: 10.1371/journal.pcbi.1005581. eCollection 2017 Jun. PLoS Comput Biol. 2017. PMID: 28574981 Free PMC article.
-
Lifelong exercise is associated with more homogeneous motor unit potential features across deep and superficial areas of vastus lateralis.Geroscience. 2021 Aug;43(4):1555-1565. doi: 10.1007/s11357-021-00356-8. Epub 2021 Mar 24. Geroscience. 2021. PMID: 33763775 Free PMC article.
-
Proof of concept for multiple nerve transfers to a single target muscle.Elife. 2021 Oct 1;10:e71312. doi: 10.7554/eLife.71312. Elife. 2021. PMID: 34596042 Free PMC article.
-
I-Spin live, an open-source software based on blind-source separation for real-time decoding of motor unit activity in humans.Elife. 2024 Oct 2;12:RP88670. doi: 10.7554/eLife.88670. Elife. 2024. PMID: 39356736 Free PMC article.
References
-
- Basmajian J. Stecko G. A new bipolar electrode for electromyography. J Appl Physiol. 1962;17:849.
-
- Bodine-Fowler S, Garfinkel A, Roy RR. Edgerton VR. Spatial distribution of muscle fibers within the territory of a motor unit. Muscle and Nerve. 1990;13:1133–1145. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

