NUMB phosphorylation destabilizes p53 and promotes self-renewal of tumor-initiating cells by a NANOG-dependent mechanism in liver cancer
- PMID: 26174965
- PMCID: PMC4618247
- DOI: 10.1002/hep.27987
NUMB phosphorylation destabilizes p53 and promotes self-renewal of tumor-initiating cells by a NANOG-dependent mechanism in liver cancer
Abstract
Stem cell populations are maintained through self-renewing divisions in which one daughter cell commits to a particular fate whereas the other retains the multipotent characteristics of its parent. The NUMB, a tumor suppressor, in conjunction with another tumor-suppressor protein, p53, preserves this property and acts as a barrier against deregulated expansion of tumor-associated stem cells. In this context, NUMB-p53 interaction plays a crucial role to maintain the proper homeostasis of both stem cells, as well as differentiated cells. Because the molecular mechanism governing the assembly and stability of the NUMB-p53 interaction/complex are poorly understood, we tried to identify the molecule(s) that govern this process. Using cancer cell lines, tumor-initiating cells (TICs) of liver, the mouse model, and clinical samples, we identified that phosphorylations of NUMB destabilize p53 and promote self-renewal of TICs in a pluripotency-associated transcription factor NANOG-dependent manner. NANOG phosphorylates NUMB by atypical protein kinase C zeta (aPKCζ), through the direct induction of Aurora A kinase (AURKA) and the repression of an aPKCζ inhibitor, lethal (2) giant larvae. By radioactivity-based kinase activity assays, we showed that NANOG enhances kinase activities of both AURKA and aPKCζ, an important upstream process for NUMB phosphorylation. Phosphorylation of NUMB by aPKCζ destabilizes the NUMB-p53 interaction and p53 proteolysis and deregulates self-renewal in TICs.
Conclusion: Post-translational modification of NUMB by the NANOG-AURKA-aPKCζ pathway is an important event in TIC self-renewal and tumorigenesis. Hence, the NANOG-NUMB-p53 signaling axis is an important regulatory pathway for TIC events in TIC self-renewal and liver tumorigenesis, suggesting a therapeutic strategy by targeting NUMB phosphorylation. Further in-depth in vivo and clinical studies are warranted to verify this suggestion.
© 2015 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Disclosures of Potential Conflict of Interest: Authors have nothing to disclose.
Figures

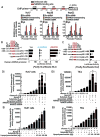
 Arrows indicate staining for respective protein. Magnifications X20 and X40. (Eii) Comparison of Immuno Recativity Score (IRS, product of percent of staining and intensity of staining) in tumor vs. non-tumor tissues. (F) Figure represents the level of NANOG and p53 proteins in NANOG transfected Huh7 cells as assessed by immunoblot analysis.
Arrows indicate staining for respective protein. Magnifications X20 and X40. (Eii) Comparison of Immuno Recativity Score (IRS, product of percent of staining and intensity of staining) in tumor vs. non-tumor tissues. (F) Figure represents the level of NANOG and p53 proteins in NANOG transfected Huh7 cells as assessed by immunoblot analysis.

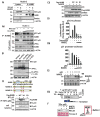

 Arrows indicate staining for respective protein. Magnifications X20 and X40. (E) Postulated mechanism of self-renewal ability through NANOG-AURKA-pNUMB pathways.
Arrows indicate staining for respective protein. Magnifications X20 and X40. (E) Postulated mechanism of self-renewal ability through NANOG-AURKA-pNUMB pathways.References
-
- Hunter T. Signaling-2000 and beyond. Cell. 2000;100:113–27. - PubMed
-
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25. - PubMed
-
- Cuesta N, Martín-Cófreces NB, Murga C, van Santen HM. Receptors, signaling networks, and disease. Sci Signal. 2011;4:mr3. - PubMed
-
- Cohen P. The twentieth century struggle to decipher insulin signalling. Nat Rev Mol Cell Biol. 2006;7:867–73. - PubMed
-
- Boulikas T. The phosphorylation connection to cancer. Int J Oncol. 1995;6:271–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
