A Phase II, Randomized, Safety and Immunogenicity Trial of a Re-Derived, Live-Attenuated Dengue Virus Vaccine in Healthy Children and Adults Living in Puerto Rico
- PMID: 26175027
- PMCID: PMC4559678
- DOI: 10.4269/ajtmh.14-0625
A Phase II, Randomized, Safety and Immunogenicity Trial of a Re-Derived, Live-Attenuated Dengue Virus Vaccine in Healthy Children and Adults Living in Puerto Rico
Abstract
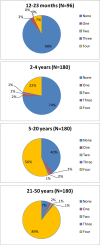
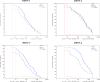
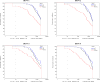
This was a double-blind, randomized, controlled, phase II clinical trial, two dose study of re-derived, live-attenuated, tetravalent dengue virus (TDEN) vaccine (two formulations) or placebo in subjects 1-50 years of age. Among the 636 subjects enrolled, 331 (52%) were primed, that is, baseline seropositive to at least one dengue virus (DENV) type. Baseline seropositivity prevalence increased with age (10% [< 2 years], 26% [2-4 years], 60% [5-20 years], and 93% [21-50 years]). Safety profiles of TDEN vaccines were similar to placebo regardless of priming status. No vaccine-related serious adverse events (SAEs) were reported. Among unprimed subjects, immunogenicity (geometric mean antibody titers [GMT] and seropositivity rates) for each DENV increased substantially in both TDEN vaccine groups with at least 74.6% seropositive for four DENV types. The TDEN vaccine candidate showed an acceptable safety and immunogenicity profile in children and adults ranging from 1 to 50 years of age, regardless of priming status. ClinicalTrials.gov: NCT00468858.
© The American Society of Tropical Medicine and Hygiene.
Figures

References
-
- Centers for Disease Control and Prevention (CDC) Dengue World Health Organization (WHO) Technical Report Series, No. 932, 2006 Annex 1. Guidelines for the Production and Quality Control of Candidate Tetravalent Dengue Virus Vaccines (Live) 2012. http://www.cdc.gov/dengue/ Available at. Accessed October 8, 2013.
-
- World Health Organization (WHO) Global Strategy for Dengue Prevention and Control, 2012–2020. WHO Report. Geneva; Switzerland: 2012. WHO/HTM/NTD/VEM/2012.5.
-
- Knope K, National Arbovirus and Malaria Advisory Committee. Giele C. Increasing notifications of dengue in Australia related to overseas travel, 1991 to 2012. Commun Dis Intell Q Rep. 2013;37:e55–e59. - PubMed
-
- Badurdeen S, Valladares DB, Farrar J, Gozzer E, Kroeger A, Kuswara N, Ranzinger SR, Tinh HT, Leite P, Mahendradhata Y, Skewes R, Verrall A. European Union, World Health Organization (WHO-TDR) supported IDAMS study group Sharing experiences: towards an evidence based model of dengue surveillance and outbreak response in Latin America and Asia. BMC Public Health. 2013;13:607. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

