The multiple roles of epidermal growth factor repeat O-glycans in animal development
- PMID: 26175457
- PMCID: PMC4551148
- DOI: 10.1093/glycob/cwv052
The multiple roles of epidermal growth factor repeat O-glycans in animal development
Abstract
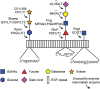
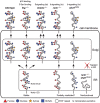
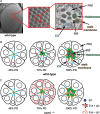
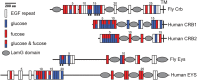
The epidermal growth factor (EGF)-like repeat is a common, evolutionarily conserved motif found in secreted proteins and the extracellular domain of transmembrane proteins. EGF repeats harbor six cysteine residues which form three disulfide bonds and help generate the three-dimensional structure of the EGF repeat. A subset of EGF repeats harbor consensus sequences for the addition of one or more specific O-glycans, which are initiated by O-glucose, O-fucose or O-N-acetylglucosamine. These glycans are relatively rare compared to mucin-type O-glycans. However, genetic experiments in model organisms and cell-based assays indicate that at least some of the glycosyltransferases involved in the addition of O-glycans to EGF repeats play important roles in animal development. These studies, combined with state-of-the-art biochemical and structural biology experiments have started to provide an in-depth picture of how these glycans regulate the function of the proteins to which they are linked. In this review, we will discuss the biological roles assigned to EGF repeat O-glycans and the corresponding glycosyltransferases. Since Notch receptors are the best studied proteins with biologically-relevant O-glycans on EGF repeats, a significant part of this review is devoted to the role of these glycans in the regulation of the Notch signaling pathway. We also discuss recently identified proteins other than Notch which depend on EGF repeat glycans to function properly. Several glycosyltransferases involved in the addition or elongation of O-glycans on EGF repeats are mutated in human diseases. Therefore, mechanistic understanding of the functional roles of these carbohydrate modifications is of interest from both basic science and translational perspectives.
Keywords: EGF repeat; Notch signaling; O-glycan; developmental biology; protein folding.
© The Author 2015. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Abd El-Aziz MM, O'Driscoll CA, Kaye RS, Barragan I, El-Ashry MF, Borrego S, Antinolo G, Pang CP, Webster AR, Bhattacharya SS. 2010. Identification of novel mutations in the ortholog of drosophila eyes shut gene (EYS) causing autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci. 51:4266–4272. - PubMed
-
- Alfaro JF, Gong CX, Monroe ME, Aldrich JT, Clauss TR, Purvine SO, Wang Z, Camp DG 2nd, Shabanowitz J, Stanley P et al. . 2012. Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc Natl Acad Sci USA. 109:7280–7285. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

