Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract
- PMID: 26175494
- PMCID: PMC4858190
- DOI: 10.1126/scisignal.aaa7540
Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract
Abstract
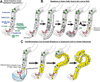
It has long been known that differentiated cells can switch fates, especially in vitro, but only recently has there been a critical mass of publications describing the mechanisms adult, postmitotic cells use in vivo to reverse their differentiation state. We propose that this sort of cellular reprogramming is a fundamental cellular process akin to apoptosis or mitosis. Because reprogramming can invoke regenerative cells from mature cells, it is critical to the long-term maintenance of tissues like the pancreas, which encounter large insults during adulthood but lack constitutively active adult stem cells to repair the damage. However, even in tissues with adult stem cells, like the stomach and intestine, reprogramming may allow mature cells to serve as reserve ("quiescent") stem cells when normal stem cells are compromised. We propose that the potential downside to reprogramming is that it increases risk for cancers that occur late in adulthood. Mature, long-lived cells may have years of exposure to mutagens. Mutations that affect the physiological function of differentiated, postmitotic cells may lead to apoptosis, but mutations in genes that govern proliferation might not be selected against. Hence, reprogramming with reentry into the cell cycle might unmask those mutations, causing an irreversible progenitor-like, proliferative state. We review recent evidence showing that reprogramming fuels irreversible metaplastic and precancerous proliferation in the stomach and pancreas. Finally, we illustrate how we think reprogrammed differentiated cells are likely candidates as cells of origin for cancers of the intestine.
Copyright © 2015, American Association for the Advancement of Science.
Figures


References
-
- Waddington CH. The Strategy of the Genes. A Discussion of Some Aspects of Theoretical Biology. London: Geoge Allen & Unwin; 1957.
-
- Mosher C. Observation on evisceration and visceral regeneration in the sea-cucumber, Actinopyga agassizi Selenka. Zoologica (NY) 1956;41:17–26.
-
- Zipori D. The stem state: plasticity is essential, whereas self-renewal and hierarchy are optional. Stem Cells. 2005;23:719–726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

