Ethylene and the Regulation of Physiological and Morphological Responses to Nutrient Deficiencies
- PMID: 26175512
- PMCID: PMC4577413
- DOI: 10.1104/pp.15.00708
Ethylene and the Regulation of Physiological and Morphological Responses to Nutrient Deficiencies
Abstract
To cope with nutrient deficiencies, plants develop both morphological and physiological responses. The regulation of these responses is not totally understood, but some hormones and signaling substances have been implicated. It was suggested several years ago that ethylene participates in the regulation of responses to iron and phosphorous deficiency. More recently, its role has been extended to other deficiencies, such as potassium, sulfur, and others. The role of ethylene in so many deficiencies suggests that, to confer specificity to the different responses, it should act through different transduction pathways and/or in conjunction with other signals. In this update, the data supporting a role for ethylene in the regulation of responses to different nutrient deficiencies will be reviewed. In addition, the results suggesting the action of ethylene through different transduction pathways and its interaction with other hormones and signaling substances will be discussed.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures

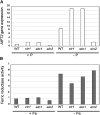

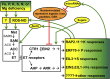
References
-
- Benlloch-González M, Romera J, Cristescu S, Harren F, Fournier JM, Benlloch M (2010) K+ starvation inhibits water-stress-induced stomatal closure via ethylene synthesis in sunflower plants. J Exp Bot 61: 1139–1145 - PubMed
-
- Borch K, Bouma TJ, Lynch JP, Brown KM (1999) Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ 22: 425–431
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

