Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders
- PMID: 26175690
- PMCID: PMC4485156
- DOI: 10.3389/fphys.2015.00187
Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders
Abstract
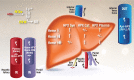
The goal here is to describe our current understanding of heme metabolism and the deleterious effects of "free" heme on immunological processes, endothelial function, systemic inflammation, and various end-organ tissues (e.g., kidney, lung, liver, etc.), with particular attention paid to the role of hemopexin (HPX). Because heme toxicity is the impetus for much of the pathology in sepsis, sickle cell disease (SCD), and other hemolytic conditions, the biological importance and clinical relevance of HPX, the predominant heme binding protein, is reinforced. A perspective on the function of HPX and haptoglobin (Hp) is presented, updating how these two proteins and their respective receptors act simultaneously to protect the body in clinical conditions that entail hemolysis and/or systemic intravascular (IVH) inflammation. Evidence from longitudinal studies in patients supports that HPX plays a Hp-independent role in genetic and non-genetic hemolytic diseases without the need for global Hp depletion. Evidence also supports that HPX has an important role in the prognosis of complex illnesses characterized predominantly by the presence of hemolysis, such as SCD, sepsis, hemolytic-uremic syndrome, and conditions involving IVH and extravascular hemolysis (EVH), such as that generated by extracorporeal circulation during cardiopulmonary bypass (CPB) and from blood transfusions. We propose that quantitating the amounts of plasma heme, HPX, Hb-Hp, heme-HPX, and heme-albumin levels in various disease states may aid in the diagnosis and treatment of the above-mentioned conditions, which is crucial to developing targeted plasma protein supplementation (i.e., "replenishment") therapies for patients with heme toxicity due to HPX depletion.
Keywords: erythrophagocytosis; haptoglobin; heme; hemolysis; hemolytic index; hemopexin; iron; plasma protein therapeutics.
Figures
References
-
- Alam J., Smith A. (1989). Receptor-mediated transport of heme by hemopexin regulates gene expression in mammalian cells. J. Biol. Chem. 264, 17637–17640. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

