Rapid Knockout and Reporter Mouse Line Generation and Breeding Colony Establishment Using EUCOMM Conditional-Ready Embryonic Stem Cells: A Case Study
- PMID: 26175717
- PMCID: PMC4485191
- DOI: 10.3389/fendo.2015.00105
Rapid Knockout and Reporter Mouse Line Generation and Breeding Colony Establishment Using EUCOMM Conditional-Ready Embryonic Stem Cells: A Case Study
Abstract
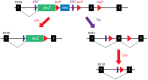
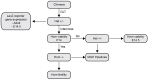
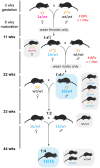
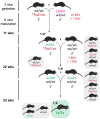
As little as a decade ago, generation of a single knockout mouse line was an expensive and time-consuming undertaking available to relatively few researchers. The International Knockout Mouse Consortium, established in 2007, has revolutionized the use of such models by creating an open-access repository of embryonic stem (ES) cells that, through sequential breeding with first FLP1 recombinase and then Cre recombinase transgenic mice, facilitates germline global or conditional deletion of almost every gene in the mouse genome. In this Case Study, we describe our experience using the repository to create mouse lines for a variety of experimental purposes. Specifically, we discuss the process of obtaining germline transmission of two European Conditional Mouse Mutagenesis Program (EUCOMM) "knockout-first" gene targeted constructs and the advantages and pitfalls of using this system. We then outline our breeding strategy and the outcomes of our efforts to generate global and conditional knockouts and reporter mice for the genes of interest. Line maintenance, removal of recombinase transgenes, and cryopreservation are also considered. Our approach led to the generation of heterozygous knockout mice within 6 months of commencing breeding to the founder mice. By describing our experiences with the EUCOMM ES cells and subsequent breeding steps, we hope to assist other researchers with the application of this valuable approach to generating versatile knockout mouse lines.
Keywords: C57BL/6; Cre recombinase; EUCOMM; Flp recombinase; conditional knockout; inducible knockout; mouse model.
Figures



References
-
- Suckow MA, Danneman P, Brayton C. First ed In: Suckow MA, editor. The Laboratory Mouse. CRC Press; (2001). p. 1–5.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

