The molecular dimension of microbial species: 2. Synechococcus strains representative of putative ecotypes inhabiting different depths in the Mushroom Spring microbial mat exhibit different adaptive and acclimative responses to light
- PMID: 26175719
- PMCID: PMC4484337
- DOI: 10.3389/fmicb.2015.00626
The molecular dimension of microbial species: 2. Synechococcus strains representative of putative ecotypes inhabiting different depths in the Mushroom Spring microbial mat exhibit different adaptive and acclimative responses to light
Abstract
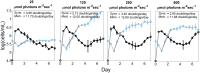
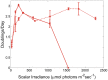
Closely related strains of thermophilic Synechococcus were cultivated from the microbial mats found in the effluent channels of Mushroom Spring, Yellowstone National Park (YNP). These strains have identical or nearly identical 16S rRNA sequences but are representative of separate, predicted putative ecotype (PE) populations, which were identified by using the more highly resolving psaA locus and which predominate at different vertical positions within the 1-mm-thick upper-green layer of the mat. Pyrosequencing confirmed that each strain contained a single, predominant psaA genotype. Strains differed in growth rate as a function of irradiance. A strain with a psaA genotype corresponding to a predicted PE that predominates near the mat surface grew fastest at high irradiances, whereas strains with psaA genotypes representative of predominant subsurface populations grew faster at low irradiance and exhibited greater sensitivity to abrupt shifts to high light. The high-light-adapted and low-light-adapted strains also exhibited differences in pigment content and the composition of the photosynthetic apparatus (photosystem ratio) when grown under different light intensities. Cells representative of the different strains had similar morphologies under low-light conditions, but under high-light conditions, cells of low-light-adapted strains became elongated and formed short chains of cells. Collectively, the results presented here are consistent with the hypothesis that closely related, but distinct, ecological species of Synechococcus occupy different light niches in the Mushroom Spring microbial mat and acclimate differently to changing light environments.
Keywords: cyanobacteria; light acclimation; light adaptation; microbial species; photosynthesis.
Figures




Similar articles
-
The molecular dimension of microbial species: 3. Comparative genomics of Synechococcus strains with different light responses and in situ diel transcription patterns of associated putative ecotypes in the Mushroom Spring microbial mat.Front Microbiol. 2015 Jun 23;6:604. doi: 10.3389/fmicb.2015.00604. eCollection 2015. Front Microbiol. 2015. PMID: 26157428 Free PMC article.
-
The molecular dimension of microbial species: 1. Ecological distinctions among, and homogeneity within, putative ecotypes of Synechococcus inhabiting the cyanobacterial mat of Mushroom Spring, Yellowstone National Park.Front Microbiol. 2015 Jun 22;6:590. doi: 10.3389/fmicb.2015.00590. eCollection 2015. Front Microbiol. 2015. PMID: 26157420 Free PMC article.
-
Relationship between Microorganisms Inhabiting Alkaline Siliceous Hot Spring Mat Communities and Overflowing Water.Appl Environ Microbiol. 2020 Nov 10;86(23):e00194-20. doi: 10.1128/AEM.00194-20. Print 2020 Nov 10. Appl Environ Microbiol. 2020. PMID: 32978131 Free PMC article.
-
Effect of temperature and light on growth of and photosynthesis by Synechococcus isolates typical of those predominating in the octopus spring microbial mat community of Yellowstone National Park.Appl Environ Microbiol. 2006 Jan;72(1):544-50. doi: 10.1128/AEM.72.1.544-550.2006. Appl Environ Microbiol. 2006. PMID: 16391090 Free PMC article.
-
Genomics, environmental genomics and the issue of microbial species.Heredity (Edinb). 2008 Feb;100(2):207-19. doi: 10.1038/sj.hdy.6801011. Epub 2007 Jun 6. Heredity (Edinb). 2008. PMID: 17551524 Review.
Cited by
-
Characterization of cyanobacterial allophycocyanins absorbing far-red light.Photosynth Res. 2020 Sep;145(3):189-207. doi: 10.1007/s11120-020-00775-2. Epub 2020 Jul 24. Photosynth Res. 2020. PMID: 32710194
-
Limiting factors in the operation of photosystems I and II in cyanobacteria.Microb Biotechnol. 2024 Aug;17(8):e14519. doi: 10.1111/1751-7915.14519. Microb Biotechnol. 2024. PMID: 39101352 Free PMC article. Review.
-
Biogeography of American Northwest Hot Spring A/B'-Lineage Synechococcus Populations.Front Microbiol. 2020 Feb 24;11:77. doi: 10.3389/fmicb.2020.00077. eCollection 2020. Front Microbiol. 2020. PMID: 32153516 Free PMC article.
-
The structural basis for light harvesting in organisms producing phycobiliproteins.Plant Cell. 2024 Oct 3;36(10):4036-4064. doi: 10.1093/plcell/koae126. Plant Cell. 2024. PMID: 38652697 Free PMC article. Review.
-
The structural basis of far-red light absorbance by allophycocyanins.Photosynth Res. 2021 Jan;147(1):11-26. doi: 10.1007/s11120-020-00787-y. Epub 2020 Oct 14. Photosynth Res. 2021. PMID: 33058014
References
-
- Allewalt J. P., Bateson M. M., Revsbech N. P., Slack K., Ward D. M. (2006). Effect of temperature and light on growth of and photosynthesis by Synechococcus isolates typical of those predominating in the Octopus Spring microbial mat community of Yellowstone National Park. Appl. Environ. Microbiol. 72 544–550. 10.1128/AEM.72.1.544-550.2006 - DOI - PMC - PubMed
-
- Becraft E. D., Wood J. M., Rusch D. B., Kühl M., Jensen S. I., Bryant D. A., et al. (2015). The molecular dimension of microbial species: 1. Ecological distinctions among, and homogeneity within, putative ecotypes of Synechococcus inhabiting the cyanobacterial mat of Mushroom Spring, Yellowstone National Park. Front. Microbiol. 6:590 10.3389/fmicb.2015.00590 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

