B Cells and Functional Antibody Responses to Combat Influenza
- PMID: 26175732
- PMCID: PMC4485180
- DOI: 10.3389/fimmu.2015.00336
B Cells and Functional Antibody Responses to Combat Influenza
Abstract
Vaccination against influenza is the most effective way to protect the population. Current vaccines provide protection by stimulating functional B- and T-cell responses; however, they are poorly immunogenic in particular segments of the population and need to be reformulated almost every year due to the genetic instability of the virus. Next-generation influenza vaccines should be designed to induce cross-reactivity, confer protection against pandemic outbreaks, and promote long-lasting immune responses among individuals at higher risk of infection. Multiple strategies are being developed for the induction of broad functional humoral immunity, including the use of adjuvants, heterologous prime-boost strategies, and epitope-based antigen design. The basic approach is to mimic natural responses to influenza virus infection by promoting cross-reactive neutralizing antibodies that directly prevent the infection. This review provides an overview of the mechanisms underlying humoral responses to influenza vaccination or natural infection, and discusses promising strategies to control influenza virus.
Keywords: functional antibody responses; hemagglutinin; influenza; neutralizing antibodies; universal influenza vaccine; vaccination strategies.
Figures
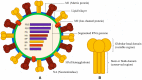
Similar articles
-
A Bivalent Heterologous DNA Virus-Like-Particle Prime-Boost Vaccine Elicits Broad Protection against both Group 1 and 2 Influenza A Viruses.J Virol. 2017 Apr 13;91(9):e02052-16. doi: 10.1128/JVI.02052-16. Print 2017 May 1. J Virol. 2017. PMID: 28179535 Free PMC article.
-
Pan-Influenza A Protection by Prime-Boost Vaccination with Cold-Adapted Live-Attenuated Influenza Vaccine in a Mouse Model.Front Immunol. 2018 Feb 1;9:116. doi: 10.3389/fimmu.2018.00116. eCollection 2018. Front Immunol. 2018. PMID: 29449842 Free PMC article.
-
Vaccination with Vesicular Stomatitis Virus-Vectored Chimeric Hemagglutinins Protects Mice against Divergent Influenza Virus Challenge Strains.J Virol. 2015 Dec 16;90(5):2544-50. doi: 10.1128/JVI.02598-15. J Virol. 2015. PMID: 26676789 Free PMC article.
-
Strategies towards universal pandemic influenza vaccines.Expert Rev Vaccines. 2016;15(2):215-25. doi: 10.1586/14760584.2016.1115352. Epub 2015 Dec 5. Expert Rev Vaccines. 2016. PMID: 26641724 Review.
-
Call for a paradigm shift in the design of universal influenza vaccines by harnessing multiple correlates of protection.Expert Opin Drug Discov. 2020 Dec;15(12):1441-1455. doi: 10.1080/17460441.2020.1801629. Epub 2020 Aug 12. Expert Opin Drug Discov. 2020. PMID: 32783765 Review.
Cited by
-
Novel Platforms for the Development of a Universal Influenza Vaccine.Front Immunol. 2018 Mar 23;9:600. doi: 10.3389/fimmu.2018.00600. eCollection 2018. Front Immunol. 2018. PMID: 29628926 Free PMC article. Review.
-
BCG Cell Wall Skeleton As a Vaccine Adjuvant Protects Both Infant and Old-Aged Mice from Influenza Virus Infection.Biomedicines. 2021 May 5;9(5):516. doi: 10.3390/biomedicines9050516. Biomedicines. 2021. PMID: 34063125 Free PMC article.
-
To whom B cells toll extrafollicular responses.Genes Immun. 2023 Dec;24(6):285-286. doi: 10.1038/s41435-023-00226-7. Epub 2023 Dec 8. Genes Immun. 2023. PMID: 38066339 Free PMC article. No abstract available.
-
Computational design and evaluation of mRNA- and protein-based conjugate vaccines for influenza A and SARS-CoV-2 viruses.J Genet Eng Biotechnol. 2023 Nov 15;21(1):120. doi: 10.1186/s43141-023-00574-x. J Genet Eng Biotechnol. 2023. PMID: 37966525 Free PMC article.
-
Cross-Reactivity Conferred by Homologous and Heterologous Prime-Boost A/H5 Influenza Vaccination Strategies in Humans: A Literature Review.Vaccines (Basel). 2021 Dec 10;9(12):1465. doi: 10.3390/vaccines9121465. Vaccines (Basel). 2021. PMID: 34960210 Free PMC article. Review.
References
-
- Available from: http://www.who.int/en/
-
- Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet (2001) 357:1937–43.10.1016/S0140-6736(00)05066-2 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

