Sub-atomic resolution X-ray crystallography and neutron crystallography: promise, challenges and potential
- PMID: 26175905
- PMCID: PMC4491318
- DOI: 10.1107/S2052252515011239
Sub-atomic resolution X-ray crystallography and neutron crystallography: promise, challenges and potential
Abstract
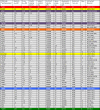
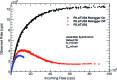
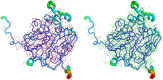
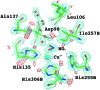
The International Year of Crystallography saw the number of macromolecular structures deposited in the Protein Data Bank cross the 100000 mark, with more than 90000 of these provided by X-ray crystallography. The number of X-ray structures determined to sub-atomic resolution (i.e. ≤1 Å) has passed 600 and this is likely to continue to grow rapidly with diffraction-limited synchrotron radiation sources such as MAX-IV (Sweden) and Sirius (Brazil) under construction. A dozen X-ray structures have been deposited to ultra-high resolution (i.e. ≤0.7 Å), for which precise electron density can be exploited to obtain charge density and provide information on the bonding character of catalytic or electron transfer sites. Although the development of neutron macromolecular crystallography over the years has been far less pronounced, and its application much less widespread, the availability of new and improved instrumentation, combined with dedicated deuteration facilities, are beginning to transform the field. Of the 83 macromolecular structures deposited with neutron diffraction data, more than half (49/83, 59%) were released since 2010. Sub-mm(3) crystals are now regularly being used for data collection, structures have been determined to atomic resolution for a few small proteins, and much larger unit-cell systems (cell edges >100 Å) are being successfully studied. While some details relating to H-atom positions are tractable with X-ray crystallography at sub-atomic resolution, the mobility of certain H atoms precludes them from being located. In addition, highly polarized H atoms and protons (H(+)) remain invisible with X-rays. Moreover, the majority of X-ray structures are determined from cryo-cooled crystals at 100 K, and, although radiation damage can be strongly controlled, especially since the advent of shutterless fast detectors, and by using limited doses and crystal translation at micro-focus beams, radiation damage can still take place. Neutron crystallography therefore remains the only approach where diffraction data can be collected at room temperature without radiation damage issues and the only approach to locate mobile or highly polarized H atoms and protons. Here a review of the current status of sub-atomic X-ray and neutron macromolecular crystallography is given and future prospects for combined approaches are outlined. New results from two metalloproteins, copper nitrite reductase and cytochrome c', are also included, which illustrate the type of information that can be obtained from sub-atomic-resolution (∼0.8 Å) X-ray structures, while also highlighting the need for complementary neutron studies that can provide details of H atoms not provided by X-ray crystallography.
Keywords: X-ray; X-ray laser; XFEL; electron transfer; hydrogen; neutron; proton; proton coupling; protonation states; radiation damage; redox biology.
Figures




Similar articles
-
Neutron macromolecular crystallography.Emerg Top Life Sci. 2018 Apr 20;2(1):39-55. doi: 10.1042/ETLS20170083. Emerg Top Life Sci. 2018. PMID: 33525781
-
Improved joint X-ray and neutron refinement procedure in Phenix.Acta Crystallogr D Struct Biol. 2023 Dec 1;79(Pt 12):1079-1093. doi: 10.1107/S2059798323008914. Epub 2023 Nov 9. Acta Crystallogr D Struct Biol. 2023. PMID: 37942718 Free PMC article.
-
Complementarity of neutron, XFEL and synchrotron crystallography for defining the structures of metalloenzymes at room temperature.IUCrJ. 2022 Jul 25;9(Pt 5):610-624. doi: 10.1107/S2052252522006418. eCollection 2022 Sep 1. IUCrJ. 2022. PMID: 36071813 Free PMC article.
-
Metalloprotein catalysis: structural and mechanistic insights into oxidoreductases from neutron protein crystallography.Acta Crystallogr D Struct Biol. 2021 Oct 1;77(Pt 10):1251-1269. doi: 10.1107/S2059798321009025. Epub 2021 Sep 27. Acta Crystallogr D Struct Biol. 2021. PMID: 34605429 Free PMC article. Review.
-
Large crystal growth by thermal control allows combined X-ray and neutron crystallographic studies to elucidate the protonation states in Aspergillus flavus urate oxidase.J R Soc Interface. 2009 Oct 6;6 Suppl 5(Suppl 5):S599-610. doi: 10.1098/rsif.2009.0162.focus. Epub 2009 Jul 8. J R Soc Interface. 2009. PMID: 19586953 Free PMC article. Review.
Cited by
-
17O NMR Spectroscopy: A Novel Probe for Characterizing Protein Structure and Folding.Biology (Basel). 2021 May 21;10(6):453. doi: 10.3390/biology10060453. Biology (Basel). 2021. PMID: 34064021 Free PMC article. Review.
-
Protein kinase A in the neutron beam: Insights for catalysis from directly observing protons.Methods Enzymol. 2020;634:311-331. doi: 10.1016/bs.mie.2019.12.003. Epub 2020 Jan 17. Methods Enzymol. 2020. PMID: 32093838 Free PMC article.
-
Electron crystallography and dedicated electron-diffraction instrumentation.Acta Crystallogr E Crystallogr Commun. 2023 Apr 14;79(Pt 5):410-422. doi: 10.1107/S2056989023003109. eCollection 2023 Apr 1. Acta Crystallogr E Crystallogr Commun. 2023. PMID: 37151820 Free PMC article.
-
Crystallography in the 21st century.IUCrJ. 2015 Nov 1;2(Pt 6):602-4. doi: 10.1107/S2052252515017509. eCollection 2015 Nov 1. IUCrJ. 2015. PMID: 26594364 Free PMC article.
-
Room Temperature Neutron Crystallography of Drug Resistant HIV-1 Protease Uncovers Limitations of X-ray Structural Analysis at 100 K.J Med Chem. 2017 Mar 9;60(5):2018-2025. doi: 10.1021/acs.jmedchem.6b01767. Epub 2017 Feb 28. J Med Chem. 2017. PMID: 28195728 Free PMC article.
References
-
- Amemiya, Y. (1997). Methods Enzymol. 276, 233–243. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

