Tumor microenvironment promotes dicarboxylic acid carrier-mediated transport of succinate to fuel prostate cancer mitochondria
- PMID: 26175936
- PMCID: PMC4497434
Tumor microenvironment promotes dicarboxylic acid carrier-mediated transport of succinate to fuel prostate cancer mitochondria
Abstract
Prostate cancer cells reprogram their metabolism, so that they support their elevated oxidative phosphorylation and promote a cancer friendly microenvironment. This work aimed to explore the mechanisms that cancer cells employ for fueling themselves with energy rich metabolites available in interstitial fluids. The mitochondria oxidative phosphorylation in metastatic prostate cancer DU145 cells and normal prostate epithelial PrEC cells were studied by high-resolution respirometry. An important finding was that prostate cancer cells at acidic pH 6.8 are capable of consuming exogenous succinate, while physiological pH 7.4 was not favorable for this process. Using specific inhibitors, it was demonstrated that succinate is transported in cancer cells by the mechanism of plasma membrane Na(+)-dependent dycarboxylic acid transporter NaDC3 (SLC13A3 gene). Although the level of expression of SLC13A3 was not significantly altered when maintaining cells in the medium with lower pH, the respirometric activity of cells under acidic condition was elevated in the presence of succinate. In contrast, normal prostate cells while expressing NaDC3 mRNA do not produce NaDC3 protein. The mechanism of succinate influx via NaDC3 in metastatic prostate cancer cells could yield a novel target for anti-cancer therapy and has the potential to be used for imaging-based diagnostics to detect non-glycolytic tumors.
Keywords: Na+-dicarboxylate transporter; Prostate cancer; acidic tumor microenvironment; mitochondria oxidative phosphorylation; succinate.
Figures
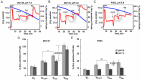


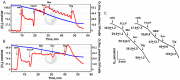

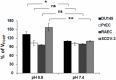
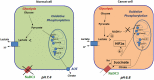
Similar articles
-
Succinate Accumulation Is Associated with a Shift of Mitochondrial Respiratory Control and HIF-1α Upregulation in PTEN Negative Prostate Cancer Cells.Int J Mol Sci. 2018 Jul 21;19(7):2129. doi: 10.3390/ijms19072129. Int J Mol Sci. 2018. PMID: 30037119 Free PMC article.
-
Transport of N-acetylaspartate by the Na(+)-dependent high-affinity dicarboxylate transporter NaDC3 and its relevance to the expression of the transporter in the brain.J Pharmacol Exp Ther. 2000 Oct;295(1):392-403. J Pharmacol Exp Ther. 2000. PMID: 10992006
-
Functional and molecular identification of sodium-coupled dicarboxylate transporters in rat primary cultured cerebrocortical astrocytes and neurons.J Neurochem. 2006 Apr;97(1):162-73. doi: 10.1111/j.1471-4159.2006.03720.x. Epub 2006 Mar 8. J Neurochem. 2006. PMID: 16524379
-
Molecular insights into prostate cancer progression: the missing link of tumor microenvironment.J Urol. 2005 Jan;173(1):10-20. doi: 10.1097/01.ju.0000141582.15218.10. J Urol. 2005. PMID: 15592017 Review.
-
SLC13 family of Na⁺-coupled di- and tri-carboxylate/sulfate transporters.Mol Aspects Med. 2013 Apr-Jun;34(2-3):299-312. doi: 10.1016/j.mam.2012.12.001. Mol Aspects Med. 2013. PMID: 23506872 Review.
Cited by
-
Mitochondria as Key Players in the Pathogenesis and Treatment of Rheumatoid Arthritis.Front Immunol. 2021 Apr 29;12:673916. doi: 10.3389/fimmu.2021.673916. eCollection 2021. Front Immunol. 2021. PMID: 33995417 Free PMC article. Review.
-
Hallmarks of Metabolic Reprogramming and Their Role in Viral Pathogenesis.Viruses. 2022 Mar 14;14(3):602. doi: 10.3390/v14030602. Viruses. 2022. PMID: 35337009 Free PMC article. Review.
-
Succinate Accumulation Is Associated with a Shift of Mitochondrial Respiratory Control and HIF-1α Upregulation in PTEN Negative Prostate Cancer Cells.Int J Mol Sci. 2018 Jul 21;19(7):2129. doi: 10.3390/ijms19072129. Int J Mol Sci. 2018. PMID: 30037119 Free PMC article.
-
Glucose oxidase and metal catalysts combined tumor synergistic therapy: mechanism, advance and nanodelivery system.J Nanobiotechnology. 2023 Oct 31;21(1):400. doi: 10.1186/s12951-023-02158-w. J Nanobiotechnology. 2023. PMID: 37907972 Free PMC article. Review.
-
Mitochondria-Mediated Anticancer Effects of Non-Thermal Atmospheric Plasma.PLoS One. 2016 Jun 6;11(6):e0156818. doi: 10.1371/journal.pone.0156818. eCollection 2016. PLoS One. 2016. PMID: 27270230 Free PMC article.
References
-
- Edlund M, Sung SY, Chung LW. Modulation of prostate cancer growth in bone microenvironments. J Cell Biochem. 2004;91:686–705. - PubMed
LinkOut - more resources
Full Text Sources
