A Frame-Shift Mutation in CAV1 Is Associated with a Severe Neonatal Progeroid and Lipodystrophy Syndrome
- PMID: 26176221
- PMCID: PMC4503302
- DOI: 10.1371/journal.pone.0131797
A Frame-Shift Mutation in CAV1 Is Associated with a Severe Neonatal Progeroid and Lipodystrophy Syndrome
Abstract
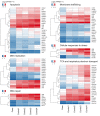
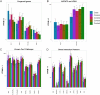
A 3-year-old female patient presenting with an unknown syndrome of a neonatal progeroid appearance, lipodystrophy, pulmonary hypertension, cutis marmorata, feeding disorder and failure to thrive was investigated by whole-genome sequencing. This revealed a de novo, heterozygous, frame-shift mutation in the Caveolin1 gene (CAV1) (p.Phe160X). Mutations in CAV1, encoding the main component of the caveolae in plasma membranes, cause Berardinelli-Seip congenital lipodystrophy type 3 (BSCL). Although BSCL is recessive, heterozygous carriers either show a reduced phenotype of partial lipodystrophy, pulmonary hypertension, or no phenotype. To investigate the pathogenic mechanisms underlying this syndrome in more depth, we performed next generation RNA sequencing of peripheral blood, which showed several dysregulated pathways in the patient that might be related to the phenotypic progeroid features (apoptosis, DNA repair/replication, mitochondrial). Secondly, we found a significant down-regulation of known Cav1 interaction partners, verifying the dysfunction of CAV1. Other known progeroid genes and lipodystrophy genes were also dysregulated. Next, western blotting of lysates of cultured fibroblasts showed that the patient shows a significantly decreased expression of wild-type CAV1 protein, demonstrating a loss-of-function mutation, though her phenotype is more severe that other heterozygotes with similar mutations. This phenotypic variety could be explained by differences in genetic background. Indications for this are supported by additional rare variants we found in AGPAT2 and LPIN1 lipodystrophy genes. CAV1, AGPAT2 and LPIN1 all play an important role in triacylglycerol (TAG) biosynthesis in adipose tissue, and the defective function in different parts of this pathway, though not all to the same extend, could contribute to a more severe lipoatrophic phenotype in this patient. In conclusion, we report, for the first time, an association of CAV1 dysfunction with a syndrome of severe premature aging and lipodystrophy. This may contribute to a better understanding of the aging process and pathogenic mechanisms that contribute to premature aging.
Conflict of interest statement
Figures

References
-
- Navarro CL, Cau P, Lévy N. Molecular bases of progeroid syndromes. Hum Mol Genet. 2006;15 Spec No: R151–61. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

