Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine administered as a two-dose schedule in adolescent girls: Five-year clinical data and modeling predictions from a randomized study
- PMID: 26176261
- PMCID: PMC4962738
- DOI: 10.1080/21645515.2015.1065363
Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine administered as a two-dose schedule in adolescent girls: Five-year clinical data and modeling predictions from a randomized study
Abstract
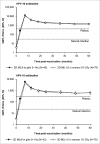
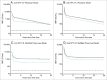
In this randomized, partially-blind study ( clinicaltrials.gov ; NCT00541970), the licensed formulation of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine (20 μg each of HPV-16/18 antigens) was found highly immunogenic up to 4 y after first vaccination, whether administered as a 2-dose (2D) schedule in girls 9-14 y or 3-dose (3D) schedule in women 15-25 y. This end-of-study analysis extends immunogenicity and safety data until Month (M) 60, and presents antibody persistence predictions estimated by piecewise and modified power law models. Healthy females (age stratified: 9-14, 15-19, 20-25 y) were randomized to receive 2D at M0,6 (N = 240 ) or 3D at M0,1,6 (N = 239). Here, results are reported for girls 9-14 y (2D) and women 15-25 y (3D). Seropositivity rates, geometric mean titers (by enzyme-linked immunosorbent assay) and geometric mean titer ratios (GMRs; 3D/2D; post-hoc exploratory analysis) were calculated. All subjects seronegative pre-vaccination in the according-to-protocol immunogenicity cohort were seropositive for anti-HPV-16 and -18 at M60. Antibody responses elicited by the 2D and 3D schedules were comparable at M60, with GMRs close to 1 (anti-HPV-16: 1.13 [95% confidence interval: 0.82-1.54]; anti-HPV-18: 1.06 [0.74-1.51]). Statistical modeling predicted that in 95% of subjects, antibodies induced by 2D and 3D schedules could persist above natural infection levels for ≥ 21 y post-vaccination. The vaccine had a clinically acceptable safety profile in both groups. In conclusion, a 2D M0,6 schedule of the HPV-16/18 AS04-adjuvanted vaccine was immunogenic for up to 5 y in 9-14 y-old girls. Statistical modeling predicted that 2D-induced antibodies could persist for longer than 20 y.
Keywords: administration schedule; female adolescents; human papillomavirus vaccine; immunogenicity; randomized controlled trial; safety; women.
Figures
References
-
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsagué X, et al.. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369: 2161-70; PMID:17602732; http://dx.doi.org/10.1016/S0140-6736(07)60946-5 - DOI - PubMed
-
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsagué X, Teixeira JC, Skinner SR, et al.. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374: 301-14; PMID:19586656; http://dx.doi.org/10.1016/S0140-6736(09)61248-4 - DOI - PubMed
-
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, Skinner SR, Apter D, Naud P, Salmeron J, et al.. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13: 89-99; PMID:22075171; http://dx.doi.org/10.1016/S1470-2045(11)70286-8 - DOI - PubMed
-
- Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmeron J, Chow SN, Apter D, Kitchener H, et al.. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13: 100-10; PMID:22075170; http://dx.doi.org/10.1016/S1470-2045(11)70287-X - DOI - PubMed
-
- Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, Dubin G, Breuer T. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008; 26: 6630-8; PMID:18845199; http://dx.doi.org/10.1016/j.vaccine.2008.09.049 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
