Safety and persistence of the humoral and cellular immune responses induced by 2 doses of an AS03-adjuvanted A(H1N1)pdm09 pandemic influenza vaccine administered to infants, children and adolescents: Two open, uncontrolled studies
- PMID: 26176592
- PMCID: PMC4635840
- DOI: 10.1080/21645515.2015.1063754
Safety and persistence of the humoral and cellular immune responses induced by 2 doses of an AS03-adjuvanted A(H1N1)pdm09 pandemic influenza vaccine administered to infants, children and adolescents: Two open, uncontrolled studies
Abstract
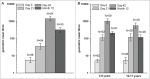
In children, 2 AS03-adjuvanted A(H1N1)pdm09 vaccine doses given 21 days apart were previously shown to induce a high humoral immune response and to have an acceptable safety profile up to 42 days following the first vaccination. Here, we analyzed the persistence data from 2 open-label studies, which assessed the safety, and humoral and cell-mediated immune responses induced by 2 doses of this vaccine. The first study was a phase II, randomized trial conducted in 104 children aged 6-35 months vaccinated with the A(H1N1)pdm09 vaccine containing 1.9 µg haemagglutinin antigen (HA) and AS03B (5.93 mg tocopherol) and the second study, a phase III, non-randomized trial conducted in 210 children and adolescents aged 3-17 years vaccinated with the A(H1N1)pdm09 vaccine containing 3.75 µg HA and AS03A (11.86 mg tocopherol). Approximately one year after the first dose, all children with available data were seropositive for haemagglutinin inhibition and neutralising antibody titres, but a decline in geometric mean antibody titres was noted. The vaccine induced a cell-mediated immune response in terms of antigen-specific CD4(+) T-cells, which persisted up to one year post-vaccination. The vaccine did not raise any safety concern, though these trials were not designed to detect rare events. In conclusion, 2 doses of the AS03-adjuvanted A(H1N1)pdm09 vaccine at 2 different dosages had a clinically acceptable safety profile, and induced high and persistent humoral and cell-mediated immune responses in children aged 6-35 months and 3-17 years. These studies have been registered at www.clinicaltrials.gov NCT00971321 and NCT00964158.
Keywords: AS03 adjuvant; H1N1 pandemic vaccine; cell-mediated immunity; children; haemagglutinin inhibition; microneutralisation; persistence; safety.
Figures




Similar articles
-
Randomized, multicenter trial of a single dose of AS03-adjuvanted or unadjuvanted H1N1 2009 pandemic influenza vaccine in children 6 months to <9 years of age: safety and immunogenicity.Pediatr Infect Dis J. 2012 Aug;31(8):848-58. doi: 10.1097/INF.0b013e31825e6cd6. Pediatr Infect Dis J. 2012. PMID: 22801094 Clinical Trial.
-
Long-Term Persistence of Cell-Mediated and Humoral Responses to A(H1N1)pdm09 Influenza Virus Vaccines and the Role of the AS03 Adjuvant System in Adults during Two Randomized Controlled Trials.Clin Vaccine Immunol. 2017 Jun 5;24(6):e00553-16. doi: 10.1128/CVI.00553-16. Print 2017 Jun. Clin Vaccine Immunol. 2017. PMID: 28446441 Free PMC article. Clinical Trial.
-
Long-term outcome of the humoral and cellular immune response of an H5N1 adjuvanted influenza vaccine in elderly persons: 2-year follow-up of a randomised open-label study.Trials. 2014 Oct 29;15:419. doi: 10.1186/1745-6215-15-419. Trials. 2014. PMID: 25354581 Free PMC article. Clinical Trial.
-
Safety of AS03-adjuvanted influenza vaccines: A review of the evidence.Vaccine. 2019 May 21;37(23):3006-3021. doi: 10.1016/j.vaccine.2019.04.048. Epub 2019 Apr 25. Vaccine. 2019. PMID: 31031030 Review.
-
Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants.J Autoimmun. 2014 May;50:1-11. doi: 10.1016/j.jaut.2014.01.033. Epub 2014 Feb 19. J Autoimmun. 2014. PMID: 24559657 Review.
Cited by
-
AS03- and MF59-Adjuvanted Influenza Vaccines in Children.Front Immunol. 2017 Dec 13;8:1760. doi: 10.3389/fimmu.2017.01760. eCollection 2017. Front Immunol. 2017. PMID: 29326687 Free PMC article. Review.
-
Antibody Persistence in Adults Two Years after Vaccination with an H1N1 2009 Pandemic Influenza Virus-Like Particle Vaccine.PLoS One. 2016 Feb 26;11(2):e0150146. doi: 10.1371/journal.pone.0150146. eCollection 2016. PLoS One. 2016. PMID: 26919288 Free PMC article. Clinical Trial.
-
Synthetic Toll-Like Receptor 4 (TLR4) and TLR7 Ligands Work Additively via MyD88 To Induce Protective Antiviral Immunity in Mice.J Virol. 2017 Sep 12;91(19):e01050-17. doi: 10.1128/JVI.01050-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724768 Free PMC article.
-
Vaccine approaches conferring cross-protection against influenza viruses.Expert Rev Vaccines. 2017 Nov;16(11):1141-1154. doi: 10.1080/14760584.2017.1379396. Epub 2017 Sep 19. Expert Rev Vaccines. 2017. PMID: 28925296 Free PMC article. Review.
-
Cell-mediated immune responses to different formulations of a live-attenuated tetravalent dengue vaccine candidate in subjects living in dengue endemic and non-endemic regions.Hum Vaccin Immunother. 2019;15(9):2090-2105. doi: 10.1080/21645515.2019.1581536. Epub 2019 Apr 15. Hum Vaccin Immunother. 2019. PMID: 30829100 Free PMC article. Clinical Trial.
References
-
- Dominguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, et al.. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009; 302:1880-7; PMID:19822626; http://dx.doi.org/10.1001/jama.2009.1536 - DOI - PubMed
-
- Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, et al.. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 2009; 324:1557-61; PMID:19433588; http://dx.doi.org/10.1126/science.1176062 - DOI - PMC - PubMed
-
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team, Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, et al.. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605-15; PMID:19423869; http://dx.doi.org/10.1056/NEJMoa0903810 - DOI - PubMed
-
- Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, et al.. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362:1708-19; PMID:20445182; http://dx.doi.org/10.1056/NEJMra1000449 - DOI - PubMed
-
- Echevarria-Zuno S, Mejia-Arangure JM, Mar-Obeso AJ, Grajales-Muniz C, Robles-Perez E, Gonzales-Leon M, Ortega-Alvarez MC, Gonzalez-Bonilla C, Rascon-Pacheco RA, Borja-Aburto VH. Infection and death from influenza A H1N1 virus in Mexico: a retrospective analysis. Lancet 2009; 374:2072-9; PMID:19913290; http://dx.doi.org/10.1016/S0140-6736(09)61638-X - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
