Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy
- PMID: 26176913
- PMCID: PMC4718588
- DOI: 10.1038/nature14658
Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy
Abstract
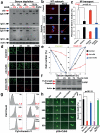
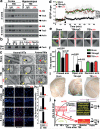
Traumatic brain injury (TBI), characterized by acute neurological dysfunction, is one of the best known environmental risk factors for chronic traumatic encephalopathy and Alzheimer's disease, the defining pathologic features of which include tauopathy made of phosphorylated tau protein (P-tau). However, tauopathy has not been detected in the early stages after TBI, and how TBI leads to tauopathy is unknown. Here we find robust cis P-tau pathology after TBI in humans and mice. After TBI in mice and stress in vitro, neurons acutely produce cis P-tau, which disrupts axonal microtubule networks and mitochondrial transport, spreads to other neurons, and leads to apoptosis. This process, which we term 'cistauosis', appears long before other tauopathy. Treating TBI mice with cis antibody blocks cistauosis, prevents tauopathy development and spread, and restores many TBI-related structural and functional sequelae. Thus, cis P-tau is a major early driver of disease after TBI and leads to tauopathy in chronic traumatic encephalopathy and Alzheimer's disease. The cis antibody may be further developed to detect and treat TBI, and prevent progressive neurodegeneration after injury.
Figures
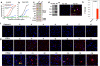
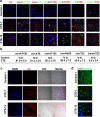
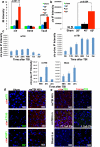
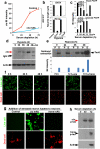
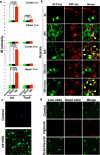

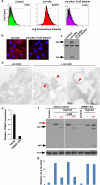
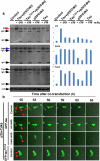
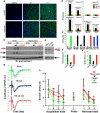
Comment in
-
Neurodegenerative diseases: A tale of two taus in traumatic brain injury.Nat Rev Drug Discov. 2015 Sep;14(9):599. doi: 10.1038/nrd4713. Nat Rev Drug Discov. 2015. PMID: 26323539 No abstract available.
References
-
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths, 2002–2006. Centers for Disease Control and Prevention [online]; 2010. http://www.cdc.gov/traumaticbraininjury/tbi_ed.html.
-
- Centers for Disease Control and Prevention CDC grand rounds: reducing severe traumatic brain injury in the United States. MMWR Morb Mortal Wkly Rep. 2013;62:549–552. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6227a6222.htm. - PMC - PubMed
-
- Tanielian T, et al. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation; Santa Monica, CA: 2008. 2008, http://www.rand.org/pubs/monographs/MG720.
-
- Omalu BI, et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA167677/CA/NCI NIH HHS/United States
- U01NS086659-01/NS/NINDS NIH HHS/United States
- R01CA167677/CA/NCI NIH HHS/United States
- R01 AG029385/AG/NIA NIH HHS/United States
- S10 RR017927/RR/NCRR NIH HHS/United States
- R01 HL111430/HL/NHLBI NIH HHS/United States
- R01AG046319/AG/NIA NIH HHS/United States
- S10RR017927/RR/NCRR NIH HHS/United States
- U01 NS086659/NS/NINDS NIH HHS/United States
- R01AG029385/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- R01 AG046319/AG/NIA NIH HHS/United States
- T32HD040128/HD/NICHD NIH HHS/United States
- T32 HD040128/HD/NICHD NIH HHS/United States
- P30AG13846/AG/NIA NIH HHS/United States
- R01HL111430/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

