The mechanism of μ-opioid receptor (MOR)-TRPV1 crosstalk in TRPV1 activation involves morphine anti-nociception, tolerance and dependence
- PMID: 26176938
- PMCID: PMC4826130
- DOI: 10.1080/19336950.2015.1069450
The mechanism of μ-opioid receptor (MOR)-TRPV1 crosstalk in TRPV1 activation involves morphine anti-nociception, tolerance and dependence
Abstract
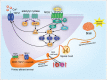
Initiated by the activation of various nociceptors, pain is a reaction to specific stimulus modalities. The μ-opioid receptor (MOR) agonists, including morphine, remain the most potent analgesics to treat patients with moderate to severe pain. However, the utility of MOR agonists is limited by the adverse effects associated with the use of these drugs, including analgesic tolerance and physical dependence. A strong connection has been suggested between the expression of the transient receptor potential vanilloid type 1 (TRPV1) ion channel and the development of inflammatory hyperalgesia. TRPV1 is important for thermal nociception induction, and is mainly expressed on sensory neurons. Recent reports suggest that opioid or TRPV1 receptor agonist exposure has contrasting consequences for anti-nociception, tolerance and dependence. Chronic morphine exposure modulates TRPV1 activation and induces the anti-nociception effects of morphine. The regulation of many downstream targets of TRPV1 plays a critical role in this process, including calcitonin gene-related peptide (CGRP) and substance P (SP). Additional factors also include capsaicin treatment blocking the anti-nociception effects of morphine in rats, as well as opioid modulation of TRPV1 responses through the cAMP-dependent PKA pathway and MAPK signaling pathways. Here, we review new insights concerning the mechanism underlying MOR-TRPV1 crosstalk and signaling pathways and discuss the potential mechanisms of morphine-induced anti-nociception, tolerance and dependence associated with the TRPV1 signaling pathway and highlight how understanding these mechanisms might help find therapeutic targets for the treatment of morphine induced antinociception, tolerance and dependence.
Keywords: calcitonin gene-related peptide (CGRP); ion channel; opioid tolerance; substance P (SP); transient receptor potential vanilloid type 1 (TRPV1); μ opioid receptor (MOR).
Figures
References
-
- Gamse R, Holzer P, Lembeck F. Indirect evidence for presynaptic location of opiate receptors on chemosensitive primary sensory neurones. Naunyn-Schmiedeberg's Arch Pharmacol 1979; 308:281-5; PMID:228212; http://dx.doi.org/10.1007/BF00501394 - DOI - PubMed
-
- Yoshimura M, North RA. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature 1983; 305:529-30; PMID:6621700; http://dx.doi.org/10.1038/305529a0 - DOI - PubMed
-
- Yaksh TL, Noueihed R. The physiology and pharmacology of spinal opiates. Ann Rev Pharmacol Toxicol 1985; 25:433-62; PMID:2988422; http://dx.doi.org/10.1146/annurev.pa.25.040185.002245 - DOI - PubMed
-
- Chen SR, Pan HL. Blocking mu opioid receptors in the spinal cord prevents the analgesic action by subsequent systemic opioids. Brain Res 2006; 1081:119-25; PMID:16499888; http://dx.doi.org/10.1016/j.brainres.2006.01.053 - DOI - PubMed
-
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, et al.. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun 2008; 22:1178-89; PMID:18599265; http://dx.doi.org/10.1016/j.bbi.2008.05.004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
