Versican V1 Overexpression Induces a Myofibroblast-Like Phenotype in Cultured Fibroblasts
- PMID: 26176948
- PMCID: PMC4503433
- DOI: 10.1371/journal.pone.0133056
Versican V1 Overexpression Induces a Myofibroblast-Like Phenotype in Cultured Fibroblasts
Abstract
Background: Versican, a chondroitin sulphate proteoglycan, is one of the key components of the provisional extracellular matrix expressed after injury. The current study evaluated the hypothesis that a versican-rich matrix alters the phenotype of cultured fibroblasts.
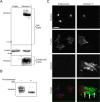
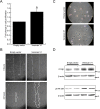
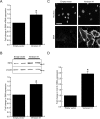
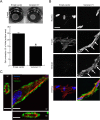
Methods and results: The full-length cDNA for the V1 isoform of human versican was cloned and the recombinant proteoglycan was expressed in murine fibroblasts. Versican expression induced a marked change in fibroblast phenotype. Functionally, the versican-expressing fibroblasts proliferated faster and displayed enhanced cell adhesion, but migrated slower than control cells. These changes in cell function were associated with greater N-cadherin and integrin β1 expression, along with increased FAK phosphorylation. The versican-expressing fibroblasts also displayed expression of smooth muscle α-actin, a marker of myofibroblast differentiation. Consistent with this observation, the versican fibroblasts displayed increased synthetic activity, as measured by collagen III mRNA expression, as well as a greater capacity to contract a collagen lattice. These changes appear to be mediated, at least in part, by an increase in active TGF-β signaling in the versican expressing fibroblasts, and this was measured by phosphorylation and nuclear accumulation of SMAD2.
Conclusions: Collectively, these data indicate versican expression induces a myofibroblast-like phenotype in cultured fibroblasts.
Conflict of interest statement
Figures


References
-
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol 1998;202: 56–66. - PubMed
-
- Geary RL, Nikkari ST, Wagner WD, Williams JK, Adams MR, Dean RH. Wound healing: a paradigm for lumen narrowing after arterial reconstruction. J Vasc Surg 1998;27: 96–106; discussion 106–108. - PubMed
-
- Farb A, Kolodgie FD, Hwang JY, Burke AP, Tefera K, Weber DK, et al. Extracellular matrix changes in stented human coronary arteries. Circulation 2004;110: 940–947. - PubMed
-
- Lin H, Wilson JE, Roberts CR, Horley KJ, Winters GL, Costanzo MR, et al. Biglycan, decorin, and versican protein expression patterns in coronary arteriopathy of human cardiac allograft: distinctness as compared to native atherosclerosis. J Heart Lung Transplant 1996;15: 1233–1247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

