Systemic and Ocular Long Pentraxin 3 in Patients with Age-Related Macular Degeneration
- PMID: 26176960
- PMCID: PMC4503310
- DOI: 10.1371/journal.pone.0132800
Systemic and Ocular Long Pentraxin 3 in Patients with Age-Related Macular Degeneration
Abstract
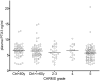
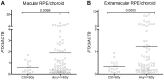
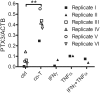
Age-related macular degeneration (AMD) has been associated with both systemic and ocular alterations of the immune system. In particular dysfunction of complement factor H (CFH), a soluble regulator of the alternative pathway of the complement system, has been implicated in AMD pathogenesis. One of the ligands for CFH is long pentraxin 3 (PTX3), which is produced locally in the retinal pigment epithelium (RPE). To test the hypothesis that PTX3 is relevant to retinal immunohomeostasis and may be associated with AMD pathogenesis, we measured plasma PTX3 protein concentration and analyzed the RPE/choroid PTX3 gene expression in patients with AMD. To measure the ability of RPE cells to secrete PTX3 in vitro, polarized ARPE-19 cells were treated with activated T cells or cytokines (interferon (IFN)-gamma and/or tumor necrosis factor (TNF)-alpha) from the basolateral side; then PTX3 protein concentration in supernatants and PTX3 gene expression in tissue lysates were quantified. Plasma levels of PTX3 were generally low and did not significantly differ between patients and controls (P=0.307). No statistically significant difference was observed between dry and exudative AMD nor was there any correlation with hsCRP or CFH genotype. The gene expression of PTX3 increased in RPE/choroid with age (P=0.0098 macular; P=0.003 extramacular), but did not differ between aged controls and AMD patients. In vitro, ARPE-19 cells increased expression of the PTX3 gene as well PTX3 apical secretions after stimulation with TNF-alpha or activated T cells (P<0.01). These findings indicate that PTX3 expressed in the eye cannot be detected systemically and systemic PTX3 may have little or no impact on disease progression, but our findings do not exclude that locally produced PTX3 produced in the posterior segment of the eye may be part of the AMD immunopathogenesis.
Conflict of interest statement
Figures
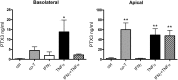
Similar articles
-
Oxidative Stress-Induced Pentraxin 3 Expression Human Retinal Pigment Epithelial Cells is Involved in the Pathogenesis of Age-Related Macular Degeneration.Int J Mol Sci. 2019 Nov 29;20(23):6028. doi: 10.3390/ijms20236028. Int J Mol Sci. 2019. PMID: 31795454 Free PMC article.
-
Human retinal pigment epithelial cells express the long pentraxin PTX3.Mol Vis. 2013;19:303-10. Epub 2013 Feb 6. Mol Vis. 2013. PMID: 23401658 Free PMC article.
-
Interferon-α coincides with suppressed levels of pentraxin-3 (PTX3) in systemic lupus erythematosus and regulates leucocyte PTX3 in vitro.Clin Exp Immunol. 2017 Jul;189(1):83-91. doi: 10.1111/cei.12957. Epub 2017 Mar 31. Clin Exp Immunol. 2017. PMID: 28257596 Free PMC article.
-
Pentraxin 3 as a prognostic biomarker in patients with systemic inflammation or infection.Mediators Inflamm. 2014;2014:421429. doi: 10.1155/2014/421429. Epub 2014 Nov 3. Mediators Inflamm. 2014. PMID: 25530683 Free PMC article. Review.
-
Pentraxin-3 and endothelial dysfunction.Adv Clin Chem. 2019;91:163-179. doi: 10.1016/bs.acc.2019.03.005. Epub 2019 May 4. Adv Clin Chem. 2019. PMID: 31331488 Review.
Cited by
-
NMOSD IgG Impact Retinal Cells in Murine Retinal Explants.Curr Issues Mol Biol. 2023 Sep 7;45(9):7319-7335. doi: 10.3390/cimb45090463. Curr Issues Mol Biol. 2023. PMID: 37754247 Free PMC article.
-
C-reactive protein and pentraxin-3 binding of factor H-like protein 1 differs from complement factor H: implications for retinal inflammation.Sci Rep. 2018 Jan 26;8(1):1643. doi: 10.1038/s41598-017-18395-7. Sci Rep. 2018. PMID: 29374201 Free PMC article.
-
High Levels of C-Reactive Protein with Low Levels of Pentraxin 3 as Biomarkers for Central Serous Chorioretinopathy.Ophthalmol Sci. 2023 Feb 3;3(3):100278. doi: 10.1016/j.xops.2023.100278. eCollection 2023 Sep. Ophthalmol Sci. 2023. PMID: 36950301 Free PMC article.
-
The Long Pentraxin PTX3 as a New Biomarker and Pharmacological Target in Age-Related Macular Degeneration and Diabetic Retinopathy.Front Pharmacol. 2022 Jan 7;12:811344. doi: 10.3389/fphar.2021.811344. eCollection 2021. Front Pharmacol. 2022. PMID: 35069222 Free PMC article. Review.
-
Monomeric C-reactive protein and inflammation in age-related macular degeneration.J Pathol. 2016 Oct;240(2):173-83. doi: 10.1002/path.4766. Epub 2016 Sep 19. J Pathol. 2016. PMID: 27376713 Free PMC article.
References
-
- Buch H, Vinding T, La CM, Appleyard M, Jensen GB, Nielsen NV (2004) Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology 111: 53–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

