Use of Ferritin Expression, Regulated by Neural Cell-Specific Promoters in Human Adipose Tissue-Derived Mesenchymal Stem Cells, to Monitor Differentiation with Magnetic Resonance Imaging In Vitro
- PMID: 26176961
- PMCID: PMC4503445
- DOI: 10.1371/journal.pone.0132480
Use of Ferritin Expression, Regulated by Neural Cell-Specific Promoters in Human Adipose Tissue-Derived Mesenchymal Stem Cells, to Monitor Differentiation with Magnetic Resonance Imaging In Vitro
Abstract
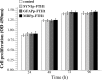
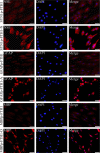
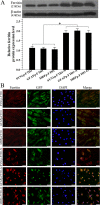
The purpose of this study was to establish a method for monitoring the neural differentiation of stem cells using ferritin transgene expression, under the control of a neural-differentiation-inducible promoter, and magnetic resonance imaging (MRI). Human adipose tissue-derived mesenchymal stem cells (hADMSCs) were transduced with a lentivirus containing the human ferritin heavy chain 1 (FTH1) gene coupled to one of three neural cell-specific promoters: human synapsin 1 promoter (SYN1p, for neurons), human glial fibrillary acidic protein promoter (GFAPp, for astrocytes), and human myelin basic protein promoter (MBPp, for oligodendrocytes). Three groups of neural-differentiation-inducible ferritin-expressing (NDIFE) hADMSCs were established: SYN1p-FTH1, GFAPp-FTH1, and MBPp-FTH1. The proliferation rate of the NDIFE hADMSCs was evaluated using a Cell Counting Kit-8 assay. Ferritin expression was assessed with western blotting and immunofluorescent staining before and after the induction of differentiation in NDIFE hADMSCs. The intracellular iron content was measured with Prussian blue iron staining and inductively coupled plasma mass spectrometry. R2 relaxation rates were measured with MRI in vitro. The proliferation rates of control and NDIFE hADMSCs did not differ significantly (P > 0.05). SYN1p-FTH1, GFAPp-FTH1, and MBPp-FTH1 hADMSCs expressed specific markers of neurons, astrocytes, and oligodendrocytes, respectively, after neural differentiation. Neural differentiation increased ferritin expression twofold, the intracellular iron content threefold, and the R2 relaxation rate two- to threefold in NDIFE hADMSCs, resulting in notable hypointensity in T2-weighted images (P < 0.05). These results were cross-validated. Thus, a link between neural differentiation and MRI signals (R2 relaxation rate) was established in hADMSCs. The use of MRI and neural-differentiation-inducible ferritin expression is a viable method for monitoring the neural differentiation of hADMSCs.
Conflict of interest statement
Figures


Similar articles
-
MRI Tracking of SPIO- and Fth1-Labeled Bone Marrow Mesenchymal Stromal Cell Transplantation for Treatment of Stroke.Contrast Media Mol Imaging. 2019 Aug 29;2019:5184105. doi: 10.1155/2019/5184105. eCollection 2019. Contrast Media Mol Imaging. 2019. PMID: 31531004 Free PMC article.
-
In Vitro Neural Differentiation of Bone Marrow Mesenchymal Stem Cells Carrying the FTH1 Reporter Gene and Detection with MRI.Biomed Res Int. 2018 Jun 26;2018:1978602. doi: 10.1155/2018/1978602. eCollection 2018. Biomed Res Int. 2018. PMID: 30046590 Free PMC article.
-
MRI detection of the malignant transformation of stem cells through reporter gene expression driven by a tumor-specific promoter.Stem Cell Res Ther. 2021 May 12;12(1):284. doi: 10.1186/s13287-021-02359-w. Stem Cell Res Ther. 2021. PMID: 33980305 Free PMC article.
-
Relaxation induced by ferritin: a better understanding for an improved MRI iron quantification.NMR Biomed. 2004 Nov;17(7):427-32. doi: 10.1002/nbm.903. NMR Biomed. 2004. PMID: 15526352 Review.
-
An Overview of Heavy Chain Ferritin in Cancer.Front Biosci (Landmark Ed). 2023 Aug 28;28(8):182. doi: 10.31083/j.fbl2808182. Front Biosci (Landmark Ed). 2023. PMID: 37664922 Review.
Cited by
-
Ferritin heavy chain as a molecular imaging reporter gene in glioma xenografts.J Cancer Res Clin Oncol. 2017 Jun;143(6):941-951. doi: 10.1007/s00432-017-2356-z. Epub 2017 Feb 28. J Cancer Res Clin Oncol. 2017. PMID: 28247036 Free PMC article.
-
In Vivo magnetic resonance imaging of xenografted tumors using FTH1 reporter gene expression controlled by a tet-on switch.Oncotarget. 2016 Nov 29;7(48):78591-78604. doi: 10.18632/oncotarget.12519. Oncotarget. 2016. PMID: 27732930 Free PMC article.
-
Caveolin-1 downregulation promotes the dopaminergic neuron-like differentiation of human adipose-derived mesenchymal stem cells.Neural Regen Res. 2021 Apr;16(4):714-720. doi: 10.4103/1673-5374.295342. Neural Regen Res. 2021. PMID: 33063733 Free PMC article.
-
Tracking Neural Progenitor Cell Migration in the Rodent Brain Using Magnetic Resonance Imaging.Front Neurosci. 2019 Jan 11;12:995. doi: 10.3389/fnins.2018.00995. eCollection 2018. Front Neurosci. 2019. PMID: 30686969 Free PMC article. Review.
-
Using magnetic resonance relaxometry to evaluate the safety and quality of induced pluripotent stem cell-derived spinal cord progenitor cells.Stem Cell Res Ther. 2024 Dec 5;15(1):465. doi: 10.1186/s13287-024-04070-y. Stem Cell Res Ther. 2024. PMID: 39639398 Free PMC article.
References
-
- Leite C, Silva NT, Mendes S, Ribeiro A, de Faria JP, Lourenço T, et al. Differentiation of human umbilical cord matrix mesenchymal stem cells into neural-like progenitor cells and maturation into an oligodendroglial-like lineage. PLoS One. 2014; 9(10): e111059 10.1371/journal.pone.0111059 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

