Implications of Heterogeneity of Treatment Effect for Reporting and Analysis of Randomized Trials in Critical Care
- PMID: 26177009
- PMCID: PMC4642199
- DOI: 10.1164/rccm.201411-2125CP
Implications of Heterogeneity of Treatment Effect for Reporting and Analysis of Randomized Trials in Critical Care
Abstract
Randomized clinical trials (RCTs) are conducted to guide clinicians' selection of therapies for individual patients. Currently, RCTs in critical care often report an overall mean effect and selected individual subgroups. Yet work in other fields suggests that such reporting practices can be improved. Specifically, this Critical Care Perspective reviews recent work on so-called "heterogeneity of treatment effect" (HTE) by baseline risk and extends that work to examine its applicability to trials of acute respiratory failure and severe sepsis. Because patients in RCTs in critical care medicine-and patients in intensive care units-have wide variability in their risk of death, these patients will have wide variability in the absolute benefit that they can derive from a given therapy. If the side effects of the therapy are not perfectly collinear with the treatment benefits, this will result in HTE, where different patients experience quite different expected benefits of a therapy. We use simulations of RCTs to demonstrate that such HTE could result in apparent paradoxes, including: (1) positive trials of therapies that are beneficial overall but consistently harm or have little benefit to low-risk patients who met enrollment criteria, and (2) overall negative trials of therapies that still consistently benefit high-risk patients. We further show that these results persist even in the presence of causes of death unmodified by the treatment under study. These results have implications for reporting and analyzing RCT data, both to better understand how our therapies work and to improve the bedside applicability of RCTs. We suggest a plan for measurement in future RCTs in the critically ill.
Keywords: acute respiratory failure; heterogeneity of treatment effect; precision medicine; randomized clinical trials; sepsis.
Figures
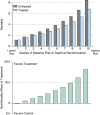
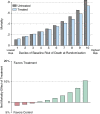
Comment in
-
Risk-based Heterogeneity of Treatment Effect in Trials and Implications for Surveillance of Clinical Effectiveness Using Regression Discontinuity Designs.Am J Respir Crit Care Med. 2015 Dec 1;192(11):1399. doi: 10.1164/rccm.201508-1533LE. Am J Respir Crit Care Med. 2015. PMID: 26623693 Free PMC article. No abstract available.
-
Reply: Risk-based Heterogeneity of Treatment Effect in Trials and Implications for Surveillance of Clinical Effectiveness Using Regression Discontinuity Designs.Am J Respir Crit Care Med. 2015 Dec 1;192(11):1399-400. doi: 10.1164/rccm.201508-1625LE. Am J Respir Crit Care Med. 2015. PMID: 26623694 Free PMC article. No abstract available.
References
-
- Guyatt GH, Sackett DL, Cook DJ. Users’ guides to the medical literature. II. How to use an article about therapy or prevention. B. What were the results and will they help me in caring for my patients? Evidence-Based Medicine Working Group. JAMA. 1994;271:59–63. - PubMed
-
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. - PubMed
-
- Rothwell PM, Warlow CP. Prediction of benefit from carotid endarterectomy in individual patients: a risk-modelling study. European Carotid Surgery Trialists’ Collaborative Group. Lancet. 1999;353:2105–2110. - PubMed
-
- Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365:82–93. - PubMed
-
- Rothwell PM. Treating individuals 2: subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

