Small molecule inhibitors of IRES-mediated translation
- PMID: 26177060
- PMCID: PMC4846101
- DOI: 10.1080/15384047.2015.1071729
Small molecule inhibitors of IRES-mediated translation
Abstract
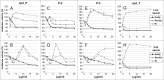
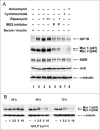
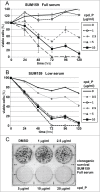
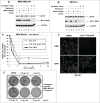
Many genes controlling cell proliferation and survival (those most important to cancer biology) are now known to be regulated specifically at the translational (RNA to protein) level. The internal ribosome entry site (IRES) provides a mechanism by which the translational efficiency of an individual or group of mRNAs can be regulated independently of the global controls on general protein synthesis. IRES-mediated translation has been implicated as a significant contributor to the malignant phenotype and chemoresistance, however there has been no effective means by which to interfere with this specialized mode of protein synthesis. A cell-based empirical high-throughput screen was performed in attempt to identify compounds capable of selectively inhibiting translation mediated through the IGF1R IRES. Results obtained using the bicistronic reporter system demonstrate selective inhibition of second cistron translation (IRES-dependent). The lead compound and its structural analogs completely block de novo IGF1R protein synthesis in genetically-unmodified cells, confirming activity against the endogenous IRES. Spectrum of activity extends beyond IGF1R to include the c-myc IRES. The small molecule IRES inhibitor differentially modulates synthesis of the oncogenic (p64) and growth-inhibitory (p67) isoforms of Myc, suggesting that the IRES controls not only translational efficiency, but also choice of initiation codon. Sustained IRES inhibition has profound, detrimental effects on human tumor cells, inducing massive (>99%) cell death and complete loss of clonogenic survival in models of triple-negative breast cancer. The results begin to reveal new insights into the inherent complexity of gene-specific translational regulation, and the importance of IRES-mediated translation to tumor cell biology.
Keywords: IGF1R; Internal ribosome entry site (IRES); c-Myc; gene-specific translational regulation; triple-negative breast cancer.
Figures




Similar articles
-
IRES inhibition induces terminal differentiation and synchronized death in triple-negative breast cancer and glioblastoma cells.Tumour Biol. 2016 Oct;37(10):13247-13264. doi: 10.1007/s13277-016-5161-4. Epub 2016 Jul 26. Tumour Biol. 2016. PMID: 27460074 Free PMC article.
-
Mechanistic Target of Rapamycin (mTOR) Inhibition Synergizes with Reduced Internal Ribosome Entry Site (IRES)-mediated Translation of Cyclin D1 and c-MYC mRNAs to Treat Glioblastoma.J Biol Chem. 2016 Jul 1;291(27):14146-14159. doi: 10.1074/jbc.M116.726927. Epub 2016 May 11. J Biol Chem. 2016. PMID: 27226604 Free PMC article.
-
Northwestern profiling of potential translation-regulatory proteins in human breast epithelial cells and malignant breast tissues: evidence for pathological activation of the IGF1R IRES.Exp Mol Pathol. 2010 Jun;88(3):341-52. doi: 10.1016/j.yexmp.2010.03.006. Epub 2010 Mar 15. Exp Mol Pathol. 2010. PMID: 20233590 Free PMC article.
-
Cis-regulatory RNA elements that regulate specialized ribosome activity.RNA Biol. 2015;12(10):1083-7. doi: 10.1080/15476286.2015.1085149. Epub 2015 Sep 1. RNA Biol. 2015. PMID: 26327194 Free PMC article. Review.
-
Targeting internal ribosome entry site (IRES)-mediated translation to block hepatitis C and other RNA viruses.FEMS Microbiol Lett. 2004 May 15;234(2):189-99. doi: 10.1016/j.femsle.2004.03.045. FEMS Microbiol Lett. 2004. PMID: 15135522 Review.
Cited by
-
Comprehensive in vivo identification of the c-Myc mRNA protein interactome using HyPR-MS.RNA. 2019 Oct;25(10):1337-1352. doi: 10.1261/rna.072157.119. Epub 2019 Jul 11. RNA. 2019. PMID: 31296583 Free PMC article.
-
Nuclear control of lung cancer cells migration, invasion and bioenergetics by eukaryotic translation initiation factor 3F.Oncogene. 2020 Jan;39(3):617-636. doi: 10.1038/s41388-019-1009-x. Epub 2019 Sep 16. Oncogene. 2020. PMID: 31527668 Free PMC article.
-
Affecting RNA biology genome-wide by binding small molecules and chemically induced proximity.Curr Opin Chem Biol. 2021 Jun;62:119-129. doi: 10.1016/j.cbpa.2021.03.006. Epub 2021 Jun 9. Curr Opin Chem Biol. 2021. PMID: 34118759 Free PMC article. Review.
-
Non-canonical translation in cancer: significance and therapeutic potential of non-canonical ORFs, m6A-modification, and circular RNAs.Cell Death Discov. 2024 Sep 27;10(1):412. doi: 10.1038/s41420-024-02185-y. Cell Death Discov. 2024. PMID: 39333489 Free PMC article. Review.
-
IRES inhibition induces terminal differentiation and synchronized death in triple-negative breast cancer and glioblastoma cells.Tumour Biol. 2016 Oct;37(10):13247-13264. doi: 10.1007/s13277-016-5161-4. Epub 2016 Jul 26. Tumour Biol. 2016. PMID: 27460074 Free PMC article.
References
-
- Baird SD, Turcotte M, Korneluk RG, Holcik M. Searching for IRES. RNA 2006; 12: 1755-85; PMID:16957278; http://dx.doi.org/ 10.1261/rna.157806 - DOI - PMC - PubMed
-
- Prats AC, Prats H. Translational control of gene expression: role of IRESs and consequences for cell transformation and angiogenesis. Prog. Nucleic Acid Res Mol Biol 2002; 72: 367-413; http://dx.doi.org/ 10.1016/S0079-6603(02)72075-8 - DOI - PubMed
-
- Marash L, Liberman N, Henis-Korenblit S, Sivan G, Reem E, Elroy-Stein O, Kimchi A. DAP5 promotes cap-independent translation of Bcl-2 and CDK1 to facilitate cell survival during mitosis. Mol Cell 2008; 30: 447-59; PMID:18450493; http://dx.doi.org/ 10.1016/j.molcel.2008.03.018 - DOI - PubMed
-
- Majumder M, Yaman I, Gaccioli F, Zeenko VV, Wang C, Caprara MG, Venema RC, Komar AA, Snider MD, Hatzoglou M. The hnRNA-binding proteins hnRNP L and PTB are required for efficient translation of the Cat-1 arginine/lysine transporter mRNA during amino acid starvation. Mol Cell Biol 2009; 29:2899-912; PMID:19273590; http://dx.doi.org/ 10.1128/MCB.01774-08 - DOI - PMC - PubMed
-
- Lang KJ, Kappel A, Goodall GJ. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol Biol Cell 2002; 13:1792-1801; PMID:12006670; http://dx.doi.org/ 10.1091/mbc.02-02-0017 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
