An Enzymatically Active β-1,3-Glucanase from Ash Pollen with Allergenic Properties: A Particular Member in the Oleaceae Family
- PMID: 26177095
- PMCID: PMC4503641
- DOI: 10.1371/journal.pone.0133066
An Enzymatically Active β-1,3-Glucanase from Ash Pollen with Allergenic Properties: A Particular Member in the Oleaceae Family
Abstract
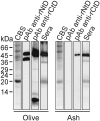
Endo-β-1,3-glucanases are widespread enzymes with glycosyl hydrolitic activity involved in carbohydrate remodelling during the germination and pollen tube growth. Although members of this protein family with allergenic activity have been reported, their effective contribution to allergy is little known. In this work, we identified Fra e 9 as a novel allergenic β-1,3-glucanase from ash pollen. We produced the catalytic and carbohydrate-binding domains as two independent recombinant proteins and characterized them from structural, biochemical and immunological point of view in comparison to their counterparts from olive pollen. We showed that despite having significant differences in biochemical activity Fra e 9 and Ole e 9 display similar IgE-binding capacity, suggesting that β-1,3-glucanases represent an heterogeneous family that could display intrinsic allergenic capacity. Specific cDNA encoding Fra e 9 was cloned and sequenced. The full-length cDNA encoded a polypeptide chain of 461 amino acids containing a signal peptide of 29 residues, leading to a mature protein of 47760.2 Da and a pI of 8.66. An N-terminal catalytic domain and a C-terminal carbohydrate-binding module are the components of this enzyme. Despite the phylogenetic proximity to the olive pollen β-1,3-glucanase, Ole e 9, there is only a 39% identity between both sequences. The N- and C-terminal domains have been produced as independent recombinant proteins in Escherichia coli and Pichia pastoris, respectively. Although a low or null enzymatic activity has been associated to long β-1,3-glucanases, the recombinant N-terminal domain has 200-fold higher hydrolytic activity on laminarin than reported for Ole e 9. The C-terminal domain of Fra e 9, a cysteine-rich compact structure, is able to bind laminarin. Both molecules retain comparable IgE-binding capacity when assayed with allergic sera. In summary, the structural and functional comparison between these two closely phylogenetic related enzymes provides novel insights into the complexity of β-1,3-glucanases, representing a heterogeneous protein family with intrinsic allergenic capacity.
Conflict of interest statement
Figures




Similar articles
-
The C-terminal domains of two homologous Oleaceae β-1,3-glucanases recognise carbohydrates differently: Laminarin binding by NMR.Arch Biochem Biophys. 2015 Aug 15;580:93-101. doi: 10.1016/j.abb.2015.07.004. Epub 2015 Jul 4. Arch Biochem Biophys. 2015. PMID: 26151774
-
The C-terminal segment of the 1,3-beta-glucanase Ole e 9 from olive (Olea europaea) pollen is an independent domain with allergenic activity: expression in Pichia pastoris and characterization.Biochem J. 2003 Feb 1;369(Pt 3):593-601. doi: 10.1042/BJ20020423. Biochem J. 2003. PMID: 12392450 Free PMC article.
-
Ole e 9, a major olive pollen allergen is a 1,3-beta-glucanase. Isolation, characterization, amino acid sequence, and tissue specificity.J Biol Chem. 2001 Jul 27;276(30):27959-66. doi: 10.1074/jbc.M103041200. Epub 2001 May 23. J Biol Chem. 2001. PMID: 11373288
-
Olive pollen recombinant allergens: value in diagnosis and immunotherapy.J Investig Allergol Clin Immunol. 2007;17 Suppl 1:4-10. J Investig Allergol Clin Immunol. 2007. PMID: 18050565 Review.
-
The spectrum of olive pollen allergens.Int Arch Allergy Immunol. 2001 Jul;125(3):185-95. doi: 10.1159/000053815. Int Arch Allergy Immunol. 2001. PMID: 11490150 Review.
Cited by
-
Perspectives of Proteomics in Respiratory Allergic Diseases.Int J Mol Sci. 2023 Aug 18;24(16):12924. doi: 10.3390/ijms241612924. Int J Mol Sci. 2023. PMID: 37629105 Free PMC article. Review.
-
Pollen Allergens for Molecular Diagnosis.Curr Allergy Asthma Rep. 2016 Apr;16(4):31. doi: 10.1007/s11882-016-0603-z. Curr Allergy Asthma Rep. 2016. PMID: 27002515 Free PMC article. Review.
-
Chemical hybridizing agent SQ-1-induced male sterility in Triticum aestivum L.: a comparative analysis of the anther proteome.BMC Plant Biol. 2018 Jan 5;18(1):7. doi: 10.1186/s12870-017-1225-x. BMC Plant Biol. 2018. PMID: 29304738 Free PMC article.
-
Safety evaluation of the food enzyme endo-1,3(4)-β-glucanase from the non-genetically modified Talaromyces versatilis strain PF8.EFSA J. 2024 Oct 9;22(10):e9033. doi: 10.2903/j.efsa.2024.9033. eCollection 2024 Oct. EFSA J. 2024. PMID: 39385970 Free PMC article.
-
Ligustrum pollen: New insights into allergic disease.World Allergy Organ J. 2020 Feb 5;13(2):100104. doi: 10.1016/j.waojou.2020.100104. eCollection 2020 Feb. World Allergy Organ J. 2020. PMID: 32055279 Free PMC article.
References
-
- El-Katatny MH, Gudelj M, Robra KH, Elnaghy MA, Gübitz GM. Characterization of a chitinase and an endo-b-1,3-glucanase from Trichoderma harzianum Rifai T24 involved in control of the phytopathogen Sclerotium rolfsii. Appl Microbiol Biotechnol. 2001;56: 137–143. - PubMed
-
- Fuchs KP, Zverlov VV, Velikodvorskaya GA, Lottspeich F, Schwarz WH. Lic16A of Clostridium thermocellum, a non-cellulosomal, highly complex endo-b-1,3-glucanase bound to the outer cell surface. Microbiology 2003;149: 1021–1031. - PubMed
-
- Verma DP and Hong Z. The ins and outs in membrane dynamics: tubulation and vesiculation. Trends Plant Sci. 2005;10: 159–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

