Chronic ethanol consumption induces mitochondrial protein acetylation and oxidative stress in the kidney
- PMID: 26177469
- PMCID: PMC4511634
- DOI: 10.1016/j.redox.2015.06.021
Chronic ethanol consumption induces mitochondrial protein acetylation and oxidative stress in the kidney
Abstract
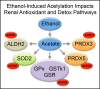
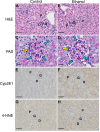
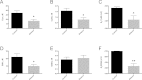
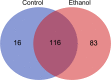
In this study, we present the novel findings that chronic ethanol consumption induces mitochondrial protein hyperacetylation in the kidney and correlates with significantly increased renal oxidative stress. A major proteomic footprint of alcoholic liver disease (ALD) is an increase in hepatic mitochondrial protein acetylation. Protein hyperacetylation has been shown to alter enzymatic function of numerous proteins and plays a role in regulating metabolic processes. Renal mitochondrial targets of hyperacetylation include numerous metabolic and antioxidant pathways, such as lipid metabolism, oxidative phosphorylation, and amino acid metabolism, as well as glutathione and thioredoxin pathways. Disruption of protein lysine acetylation has the potential to impair renal function through metabolic dysregulation and decreased antioxidant capacity. Due to a significant elevation in ethanol-mediated renal oxidative stress, we highlight the acetylation of superoxide dismutase, peroxiredoxins, glutathione reductase, and glutathione transferase enzymes. Since oxidative stress is a known factor in ethanol-induced nephrotoxicity, we examined biochemical markers of protein hyperacetylation and oxidative stress. Our results demonstrate increased protein acetylation concurrent with depleted glutathione, altered Cys redox potential, and the presence of 4-HNE protein modifications in our 6-week model of early-stage alcoholic nephrotoxicity. These findings support the hypothesis that ethanol metabolism causes an influx of mitochondrial metabolic substrate, resulting in mitochondrial protein hyperacetylation with the potential to impact mitochondrial metabolic and antioxidant processes.
Keywords: 4-Hydroxynonenal; Acetylation; Ethanol; Glutathione; Kidney; Oxidative stress.
Copyright © 2015. Published by Elsevier B.V.
Figures


References
-
- World Health Organization . World Health Organization; Geneva: 2014. Global Status Report on Alcohol and Health 2014.
-
- Vidali M., Stewart S.F., Albano E. Interplay between oxidative stress and immunity in the progression of alcohol-mediated liver injury. Trends Mol. Med. 2008;14:63–71. - PubMed
-
- Das S.K., Vasudevan D.M. Alcohol-induced oxidative stress. Life Sci. 2007;81:177–187. - PubMed
-
- Seitz H.K., Lieber C.S., Stickel F., Salaspuro M., Schlemmer H.P., Horie Y. Alcoholic liver disease: from pathophysiology to therapy. Alcohol. Clin. Exp. Res. 2005;29:1276–1281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

