Multiple Levels of Regulation of Sororin by Cdk1 and Aurora B
- PMID: 26177583
- PMCID: PMC12337688
- DOI: 10.1002/jcb.25277
Multiple Levels of Regulation of Sororin by Cdk1 and Aurora B
Abstract
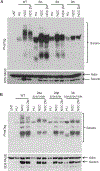
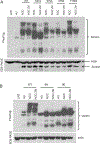
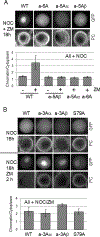
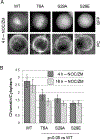
The cohesin complex holds sister chromatids together until all chromosomes are properly attached to the mitotic spindle. Cleavage of the cohesin subunit Rad21 at the metaphase to anaphase transition allows separation of sister chromatids and is fundamental for the creation of identical daughter cells. Sororin blocks removal of cohesin from chromosomes from S phase until mitosis. In mitosis, Sororin is phosphorylated by Cdk1 releasing it from the cohesin complex. Aurora B phosphorylation of Sororin may play a similar role as Cdk1. Using PhosTag electrophoresis, we detect multiple Sororin species suggesting that phosphorylation of Sororin in mitosis is heterogeneous. Mutating the Cdk1 consensus site S21 to alanine eliminates many of the phosphorylated species suggesting that S21 is a key site of phosphorylation in vivo. Inhibiting Aurora B reduces phosphorylation of Sororin in cells, but only if Cdk1 sites are intact suggesting that some phosphorylation events on Sororin may be sequential. Surprisingly, mutating Aurora B consensus sites in Sororin causes it to relocalize to the nucleolus during interphase and blocks its interaction with chromosomes in Aurora B-inhibited cells. These observations indicate that phosphorylation plays unexpected roles in regulating the subcellular localization of Sororin.
Keywords: COHESIN; MITOSIS; NUCLEOLUS; PHOSPHORYLATION; PHOSTAG.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of Interest
The authors state that they have no conflict of interest to declare.
Figures



References
-
- Barber TD, McManus K, Yuen KW, Reis M, Parmigiani G, Shen D, Barrett I, Nouhi Y, Spencer F, Markowitz S, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C, Hieter P. 2008. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci U S A 105:3443–8. - PMC - PubMed
-
- Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. 2006. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol 24:1285–92. - PubMed
-
- Cantin GT, Yi W, Lu B, Park SK, Xu T, Lee JD, Yates JR 3rd. 2008. Combining protein-based IMAC, peptide-based IMAC, and MudPIT for efficient phosphoproteomic analysis. J Proteome Res 7:1346–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

