Up-regulation of micro-RNA765 in human failing hearts is associated with post-transcriptional regulation of protein phosphatase inhibitor-1 and depressed contractility
- PMID: 26177627
- PMCID: PMC5693221
- DOI: 10.1002/ejhf.323
Up-regulation of micro-RNA765 in human failing hearts is associated with post-transcriptional regulation of protein phosphatase inhibitor-1 and depressed contractility
Abstract
Aims: Impaired sarcoplasmic reticulum (SR) Ca(2+) cycling and depressed contractility, a hallmark of human and experimental heart failure, has been partially attributed to increased protein phosphatase 1 (PP-1) activity, associated with down-regulation of its endogenous inhibitor-1. The levels and activity of inhibitor-1 are reduced in failing hearts, contributing to dephosphorylation and inactivation of key calcium cycling proteins. Therefore, we investigated the mechanisms that mediate decreases in inhibitor-1 by post-transcriptional modification.
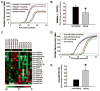
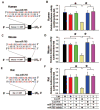
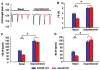
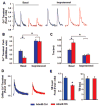
Methods and results: Bioinformatics revealed that 17 human microRNAs may serve as modulators of inhibitor-1. However, real-time PCR analysis identified only one of these microRNAs, miR-765, as being increased in human failing hearts concomitant with decreased inhibitor-1 levels. Expression of miR-765 in HEK293 cells or mouse ventricular myocytes confirmed suppression of inhibitor-1 levels through binding of this miR-765 to the 3'-untranslated region of inhibitor-1 mRNA. To determine the functional significance of miR-765 in Ca(2+) cycling, pri-miR-765 as well as a non-translated nucleotide sequence (miR-Ctrl) were expressed in adult mouse ventricular myocytes. The inhibitor-1 expression levels were decreased, accompanied by enhanced PP-1 activity in the miR-765 cardiomyocytes, and these reflected depressed contractile mechanics and Ca(2+) transients, compared with the miR-Ctrl group. The depressive effects were associated with decreases in the phosphorylation of phospholamban and SR Ca(2+) load. These miR-765 negative inotropic effects were abrogated in inhibitor-1-deficient cardiomyocytes, suggesting its apparent specificity for inhibitor-1.
Conclusions: miR-765 levels are increased in human failing hearts. Such increases may contribute to depressed cardiac function through reduced inhibitor-1 expression and enhanced PP-1 activity, associated with reduced SR Ca(2+) load.
Keywords: Calcium cycling; Cardiomyocyte contractility; Heart failure; Inhibitor-1; MicroRNA-765; Protein phosphatase 1.
© 2015 The Authors. European Journal of Heart Failure © 2015 European Society of Cardiology.
Conflict of interest statement
Figures

Similar articles
-
Enhanced myocyte contractility and Ca2+ handling in a calcineurin transgenic model of heart failure.Cardiovasc Res. 2002 Apr;54(1):105-16. doi: 10.1016/s0008-6363(02)00230-4. Cardiovasc Res. 2002. PMID: 12062367
-
Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium.J Mol Cell Cardiol. 2001 Jul;33(7):1345-53. doi: 10.1006/jmcc.2001.1394. J Mol Cell Cardiol. 2001. PMID: 11437540
-
AAV-9 mediated phosphatase-1 inhibitor-1 overexpression improves cardiac contractility in unchallenged mice but is deleterious in pressure-overload.Gene Ther. 2018 Jan;25(1):13-19. doi: 10.1038/gt.2017.97. Epub 2018 Jan 19. Gene Ther. 2018. PMID: 29350681
-
Phospholamban as a therapeutic modality in heart failure.Novartis Found Symp. 2006;274:156-71; discussion 172-5, 272-6. Novartis Found Symp. 2006. PMID: 17019811 Review.
-
[Modification of sarco-endoplasmic reticulum Ca(2 +) -ATPase in the failing cardiomyocyte].Clin Calcium. 2013 Apr;23(4):535-42. Clin Calcium. 2013. PMID: 23545743 Review. Japanese.
Cited by
-
Lack of miR-133a Decreases Contractility of Diabetic Hearts: A Role for Novel Cross Talk Between Tyrosine Aminotransferase and Tyrosine Hydroxylase.Diabetes. 2016 Oct;65(10):3075-90. doi: 10.2337/db16-0023. Epub 2016 Jul 13. Diabetes. 2016. PMID: 27411382 Free PMC article.
-
MicroRNA-765 regulates neural stem cell proliferation and differentiation by modulating Hes1 expression.Am J Transl Res. 2016 Jul 15;8(7):3115-23. eCollection 2016. Am J Transl Res. 2016. PMID: 27508032 Free PMC article.
-
The roles and mechanisms of epigenetic regulation in pathological myocardial remodeling.Front Cardiovasc Med. 2022 Aug 26;9:952949. doi: 10.3389/fcvm.2022.952949. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36093141 Free PMC article. Review.
-
Long noncoding RNA Mhrt alleviates angiotensin II-induced cardiac hypertrophy phenotypes by mediating the miR-765/Wnt family member 7B pathway.Open Med (Wars). 2023 May 12;18(1):20230681. doi: 10.1515/med-2023-0681. eCollection 2023. Open Med (Wars). 2023. PMID: 37197359 Free PMC article.
-
A novel human S10F-Hsp20 mutation induces lethal peripartum cardiomyopathy.J Cell Mol Med. 2018 Aug;22(8):3911-3919. doi: 10.1111/jcmm.13665. Epub 2018 May 15. J Cell Mol Med. 2018. PMID: 29761889 Free PMC article.
References
-
- Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. - PubMed
-
- El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res. 2004;61:87–93. - PubMed
-
- Nicolaou P, Rodriguez P, Ren X, Zhou X, Qian J, Sadayappan S, Mitton B, Pathak A, Robbins J, Hajjar RJ, Jones K, Kranias EG. Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res. 2009;104:1012–1020. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

