Orai/CRACM1 and KCa3.1 ion channels interact in the human lung mast cell plasma membrane
- PMID: 26177720
- PMCID: PMC4504158
- DOI: 10.1186/s12964-015-0112-z
Orai/CRACM1 and KCa3.1 ion channels interact in the human lung mast cell plasma membrane
Abstract
Background: Orai/CRACM1 ion channels provide the major Ca(2+) influx pathway for FcεRI-dependent human lung mast cell (HLMC) mediator release. The Ca(2+)-activated K(+) channel KCa3.1 modulates Ca(2+) influx and the secretory response through hyperpolarisation of the plasma membrane. We hypothesised that there is a close functional and spatiotemporal interaction between these Ca(2+)- and K(+)-selective channels.
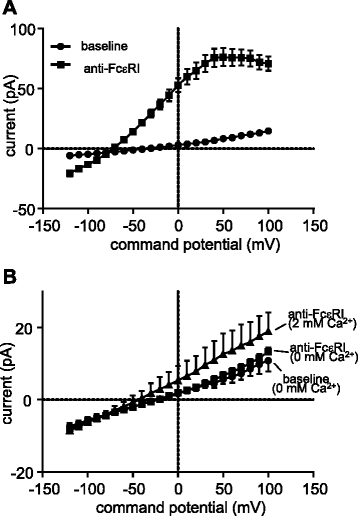
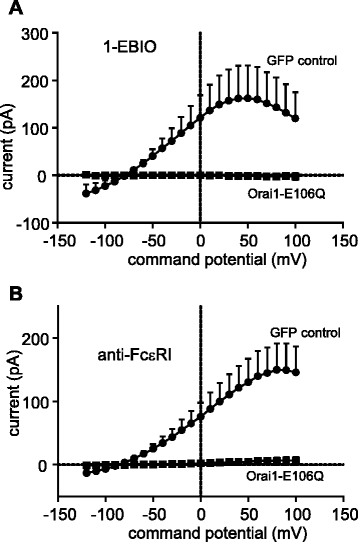
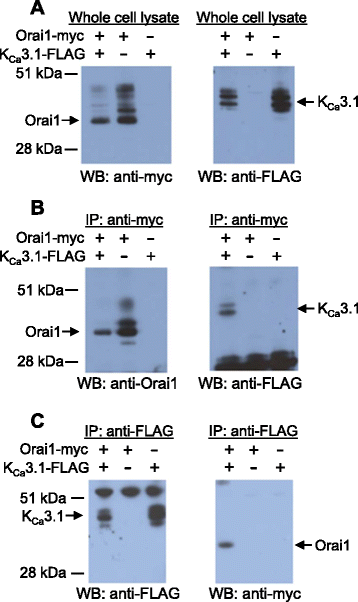
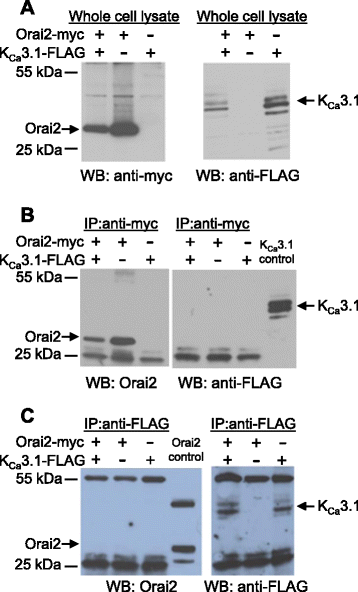
Results: Activation of FcεRI-dependent HLMC KCa3.1 currents was dependent on the presence of extracellular Ca(2+), and attenuated in the presence of the selective Orai blocker GSK-7975A. Currents elicited by the KCa3.1 opener 1-EBIO were also attenuated by GSK-7975A. The Orai1 E106Q dominant-negative mutant ablated 1-EBIO and FcεRI-dependent KCa3.1 currents in HLMCs. Orai1 but not Orai2 was shown to co-immunoprecipitate with KCa3.1 when overexpressed in HEK293 cells, and Orai1 and KCa3.1 were seen to co-localise in the HEK293 plasma membrane using confocal microscopy.
Conclusion: KCa3.1 activation in HLMCs is highly dependent on Ca(2+) influx through Orai1 channels, mediated via a close spatiotemporal interaction between the two channels.
Figures


References
-
- Church MK, Pao GJ, Holgate ST. Characterization of histamine secretion from mechanically dispersed human lung mast cells: effects of anti-IgE, calcium ionophore A23187, compound 48/80, and basic polypeptides. J Immunol. 1982;129:2116–2121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

