Odanacatib increases mineralized callus during fracture healing in a rabbit ulnar osteotomy model
- PMID: 26178170
- PMCID: PMC6680236
- DOI: 10.1002/jor.22982
Odanacatib increases mineralized callus during fracture healing in a rabbit ulnar osteotomy model
Abstract
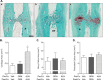
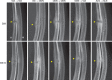
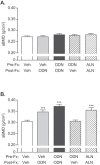
The effects of the cathepsin K inhibitor odanacatib (ODN) on fracture healing were monitored for ~6 and 15 weeks post-fracture in two separate studies using the unilateral transverse mid-ulnar osteotomy model in skeletally mature female rabbits. Rabbits were pre-treated for 3-4 weeks with vehicle (Veh), ODN (2 mg/kg, po, daily), or alendronate (ALN) (0.3 mg/kg, sc, twice-weekly) prior to osteotomy. In Study 1, the animals were maintained on the same respective treatment for ~6 weeks. In Study 2, the animals were also continued on the same therapy or switched from Veh to ODN or ODN to Veh for 15 weeks. No treatment-related impairment of fracture union was seen by qualitative histological assessments in the first study. Cartilage retention was detected in the calluses of ALN-treated rabbits at week-6, while calluses in the ODN and Veh groups contained bony tissue with significantly less residual cartilage. ODN treatment also markedly increased the number of cathepsin K-(+) osteoclasts in the callus, indicating enhanced callus remodeling. From the second study, ex vivo DXA and pQCT confirmed that ODN treatment pre- and post-osteotomy increased callus bone mineral content and bone mineral density (BMD) versus Veh (p < 0.001) and discontinuation of ODN post-surgery returned callus BMD to Veh. Peak load of ODN- or ALN-treated calluses were comparable to Veh. ODN increased callus yield load (20%, p = 0.056) and stiffness (26%, p < 0.05) versus Veh. These studies demonstrated that ODN increased mineralized callus during the early phase of fracture repair without impairing callus formation or biomechanical integrity at the fracture site.
Keywords: callus formation; cathepsin K; fracture healing; osteoclast; rabbit.
© 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures


Similar articles
-
Odanacatib, effects of 16-month treatment and discontinuation of therapy on bone mass, turnover and strength in the ovariectomized rabbit model of osteopenia.Bone. 2016 Dec;93:86-96. doi: 10.1016/j.bone.2016.09.012. Epub 2016 Sep 15. Bone. 2016. PMID: 27639811
-
Odanacatib Restores Trabecular Bone of Skeletally Mature Female Rabbits With Osteopenia but Induces Brittleness of Cortical Bone: A Comparative Study of the Investigational Drug With PTH, Estrogen, and Alendronate.J Bone Miner Res. 2016 Mar;31(3):615-29. doi: 10.1002/jbmr.2719. Epub 2015 Oct 26. J Bone Miner Res. 2016. Retraction in: J Bone Miner Res. 2015 Oct;30(10):1944. doi: 10.1002/jbmr.2520. PMID: 26391310 Retracted.
-
Effect of odanacatib on bone turnover markers, bone density and geometry of the spine and hip of ovariectomized monkeys: a head-to-head comparison with alendronate.Bone. 2013 Oct;56(2):489-96. doi: 10.1016/j.bone.2013.06.008. Epub 2013 Jun 24. Bone. 2013. PMID: 23806798
-
Potential role of odanacatib in the treatment of osteoporosis.Clin Interv Aging. 2012;7:235-47. doi: 10.2147/CIA.S26729. Epub 2012 Jul 12. Clin Interv Aging. 2012. PMID: 22866001 Free PMC article. Review.
-
Role of odanacatib in reducing bone loss due to endodontic disease: An overview.J Int Soc Prev Community Dent. 2016 Dec;6(Suppl 3):S175-S181. doi: 10.4103/2231-0762.197183. J Int Soc Prev Community Dent. 2016. PMID: 28217533 Free PMC article. Review.
Cited by
-
Cathepsin K Inhibitors for Osteoporosis: Biology, Potential Clinical Utility, and Lessons Learned.Endocr Rev. 2017 Aug 1;38(4):325-350. doi: 10.1210/er.2015-1114. Endocr Rev. 2017. PMID: 28651365 Free PMC article. Review.
-
Osteoclast depletion with clodronate liposomes delays fracture healing in mice.J Orthop Res. 2017 Aug;35(8):1699-1706. doi: 10.1002/jor.23440. Epub 2016 Oct 6. J Orthop Res. 2017. PMID: 27653179 Free PMC article.
-
From disease to treatment: from rare skeletal disorders to treatments for osteoporosis.Endocrine. 2016 Jun;52(3):414-26. doi: 10.1007/s12020-016-0888-7. Epub 2016 Feb 18. Endocrine. 2016. PMID: 26892377 Free PMC article. Review.
-
Small-molecule amines: a big role in the regulation of bone homeostasis.Bone Res. 2023 Jul 24;11(1):40. doi: 10.1038/s41413-023-00262-z. Bone Res. 2023. PMID: 37482549 Free PMC article. Review.
-
Monocyte/Macrophage Lineage Cells From Fetal Erythromyeloid Progenitors Orchestrate Bone Remodeling and Repair.Front Cell Dev Biol. 2021 Feb 4;9:622035. doi: 10.3389/fcell.2021.622035. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33614650 Free PMC article. Review.
References
-
- Gauthier JY, et al. 2008. The discovery of odanacatib (MK‐0822), a selective inhibitor of cathepsin K 1. Bioorg Med Chem Lett 18:923–928. - PubMed
-
- Gerstenfeld LC, et al. 2003. Fracture healing as a post‐natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 88:873–884. - PubMed
-
- Schindeler A, et al. 2008. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol 19:459–466. - PubMed
-
- Barnes GL, et al. 1999. Growth factor regulation of fracture repair. J Bone Miner Res 14:1805–1815. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

