Evaluation of Cross-Protection of a Lineage 1 West Nile Virus Inactivated Vaccine against Natural Infections from a Virulent Lineage 2 Strain in Horses, under Field Conditions
- PMID: 26178384
- PMCID: PMC4550672
- DOI: 10.1128/CVI.00302-15
Evaluation of Cross-Protection of a Lineage 1 West Nile Virus Inactivated Vaccine against Natural Infections from a Virulent Lineage 2 Strain in Horses, under Field Conditions
Abstract
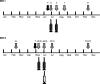
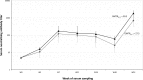
Although experimental data regarding cross-protection of horse West Nile virus (WNV) vaccines against lineage 2 infections exist, the cross-protective efficacy of these vaccines under field conditions has not been demonstrated. This study was conducted to evaluate the capability of an inactivated lineage 1 vaccine (Equip WNV) to protect against natural infections from the Nea Santa-Greece-2010 lineage 2 strain. In total, 185 WNV-seronegative horses in Thessaloniki, Greece, were selected during 2 consecutive years (2011 and 2012); 140 were immunized, and 45 were used as controls. Horses were examined for signs compatible with WNV infection. Neutralizing antibody titers against the Greek strain and the PaAn001/France lineage 1 strain were determined in immunized horses. WNV circulation was detected during both years in the study area. It was estimated that 37% and 27% of the horses were infected during 2011 and 2012, respectively. Three control animals developed clinical signs, and the WNV diagnosis was confirmed. Signs related to WNV infection were not observed in the vaccinated animals. The nonvaccinated animals had a 7.58% ± 1.82% higher chance of exhibiting signs than immunized animals (P < 0.05). Neutralizing antibodies raised against both strains in all immunized horses were detectable 1 month after the initial vaccination course. The cross-protective capacity of the lowest titer (1:40) was evident in 19 animals which were subsequently infected and did not exhibit signs. Neutralizing antibodies were detectable until the annual booster, when strong anamnestic responses were observed (geometrical mean titer ratio [GMTR] for lineage 1 of 30.2; GMTR for lineage 2 of 27.5). The results indicate that Equip WNV is capable of inducing cross-protection against natural infections from a virulent lineage 2 WNV strain in horses.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Protection provided by a recombinant ALVAC(®)-WNV vaccine expressing the prM/E genes of a lineage 1 strain of WNV against a virulent challenge with a lineage 2 strain.Vaccine. 2011 Jun 20;29(28):4608-12. doi: 10.1016/j.vaccine.2011.04.058. Epub 2011 May 5. Vaccine. 2011. PMID: 21549780
-
Serum neutralising antibody titres against a lineage 2 neuroinvasive West Nile Virus strain in response to vaccination with an inactivated lineage 1 vaccine in a European endemic area.Vet Immunol Immunopathol. 2020 Sep;227:110087. doi: 10.1016/j.vetimm.2020.110087. Epub 2020 Jun 20. Vet Immunol Immunopathol. 2020. PMID: 32629300
-
Protection of horses from West Nile virus Lineage 2 challenge following immunization with a whole, inactivated WNV lineage 1 vaccine.Vaccine. 2014 Sep 22;32(42):5455-9. doi: 10.1016/j.vaccine.2014.07.093. Epub 2014 Aug 12. Vaccine. 2014. PMID: 25131745
-
West Nile Virus: is a vaccine needed?Curr Opin Investig Drugs. 2010 Feb;11(2):139-46. Curr Opin Investig Drugs. 2010. PMID: 20112163 Review.
-
Comparison of assays for the detection of West Nile virus antibodies in equine serum after natural infection or vaccination.Vet Immunol Immunopathol. 2017 Jan;183:1-6. doi: 10.1016/j.vetimm.2016.10.015. Epub 2016 Nov 3. Vet Immunol Immunopathol. 2017. PMID: 28063471 Review.
Cited by
-
Pathogenicity and virulence of West Nile virus revisited eight decades after its first isolation.Virulence. 2021 Dec;12(1):1145-1173. doi: 10.1080/21505594.2021.1908740. Virulence. 2021. PMID: 33843445 Free PMC article.
-
Seroprevalence and Risk Factors for Equine West Nile Virus Infections in Eastern Germany, 2020.Viruses. 2022 May 30;14(6):1191. doi: 10.3390/v14061191. Viruses. 2022. PMID: 35746662 Free PMC article.
-
Lessons Learned from West Nile Virus Infection:Vaccinations in Equines and Their Implications for One Health Approaches.Viruses. 2024 May 14;16(5):781. doi: 10.3390/v16050781. Viruses. 2024. PMID: 38793662 Free PMC article. Review.
-
Animal and Human Vaccines against West Nile Virus.Pathogens. 2020 Dec 21;9(12):1073. doi: 10.3390/pathogens9121073. Pathogens. 2020. PMID: 33371384 Free PMC article. Review.
-
European College of Equine Internal Medicine consensus statement on equine flaviviridae infections in Europe.J Vet Intern Med. 2022 Nov;36(6):1858-1871. doi: 10.1111/jvim.16581. Epub 2022 Nov 11. J Vet Intern Med. 2022. PMID: 36367340 Free PMC article.
References
-
- Rappole JH, Hubálek Z. 2003. Migratory birds and West Nile virus. J Appl Microbiol 94(Suppl):47S–58S. - PubMed
-
- Linke S, Niedrig M, Kaiser A, Ellerbrok H, Müller K, Müller T, Conraths FJ, Mühle RU, Schmidt D, Köppen U, Bairlein F, Berthold P, Pauli G. 2007. Serologic evidence of West Nile virus infections in wild birds captured in Germany. Am J Trop Med Hyg 77:358–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

