Human hepatocyte assessment of imatinib drug-drug interactions - complexities in clinical translation
- PMID: 26178713
- PMCID: PMC4631182
- DOI: 10.1111/bcp.12723
Human hepatocyte assessment of imatinib drug-drug interactions - complexities in clinical translation
Abstract
Aim: Inducers and inhibitors of CYP3A, such as ritonavir and efavirenz, may be used as part of the highly active antiretroviral therapy (HAART) to treat HIV patients. HIV patients with chronic myeloid leukemia or gastrointestinal stromal tumour may need imatinib, a CYP3A4 substrate with known exposure response-relationships. Administration of imatinib to patients on ritonavir or efavirenz may result in altered imatinib exposure leading to increased toxicity or failure of therapy, respectively. We used primary human hepatocyte cultures to evaluate the magnitude of interaction between imatinib and ritonavir/efavirenz.
Methods: Hepatocytes were pre-treated with vehicle, ritonavir, ketoconazole, efavirenz or rifampicin, and the metabolism of imatinib was characterized over time. Concentrations of imatinib and metabolite were quantitated in combined lysate and medium, using LC-MS.
Results: The predicted changes in imatinib CLoral (95% CI) with ketoconazole, ritonavir, rifampicin and efavirenz were 4.0-fold (0, 9.2) lower, 2.8-fold (0.04, 5.5) lower, 2.9-fold (2.2, 3.5) higher and 2.0-fold (0.42, 3.5) higher, respectively. These predictions were in good agreement with clinical single dose drug-drug interaction studies, but not with reports of imatinib interactions at steady-state. Alterations in metabolism were similar after acute or chronic imatinib exposure.
Conclusions: In vitro human hepatocytes predicted increased clearance of imatinib with inducers and decreased clearance with inhibitors of CYP enzymes. The impact of HAART on imatinib may depend on whether it is being initiated or has already been dosed chronically in patients. Therapeutic drug monitoring may have a role in optimizing imatinib therapy in this patient population.
Keywords: HIV drugs; drug-drug interactions; human hepatocytes; imatinib.
© 2015 The British Pharmacological Society.
Figures

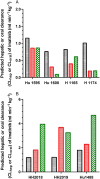
 ), ritonavir 10 μ
), ritonavir 10 μ ) and ketoconazole (
) and ketoconazole ( ) 10 μ
) 10 μ ), efavirenz 10 μ
), efavirenz 10 μ ) and rifampicin 10 μ
) and rifampicin 10 μ ) (B)
) (B)
Similar articles
-
Ritonavir and efavirenz significantly alter the metabolism of erlotinib--an observation in primary cultures of human hepatocytes that is relevant to HIV patients with cancer.Drug Metab Dispos. 2013 Oct;41(10):1843-51. doi: 10.1124/dmd.113.052100. Epub 2013 Aug 2. Drug Metab Dispos. 2013. PMID: 23913028 Free PMC article.
-
Potential interactions between HIV drugs, ritonavir and efavirenz and anticancer drug, nilotinib--a study in primary cultures of human hepatocytes that is applicable to HIV patients with cancer.J Clin Pharmacol. 2014 Nov;54(11):1272-9. doi: 10.1002/jcph.333. Epub 2014 May 27. J Clin Pharmacol. 2014. PMID: 24846165
-
Preclinical assessment of the interactions between the antiretroviral drugs, ritonavir and efavirenz, and the tyrosine kinase inhibitor erlotinib.Cancer Chemother Pharmacol. 2015 Oct;76(4):813-9. doi: 10.1007/s00280-015-2856-y. Epub 2015 Sep 2. Cancer Chemother Pharmacol. 2015. PMID: 26330331 Free PMC article.
-
Lurasidone drug-drug interaction studies: a comprehensive review.Drug Metabol Drug Interact. 2014;29(3):191-202. doi: 10.1515/dmdi-2014-0005. Drug Metabol Drug Interact. 2014. PMID: 24825095 Review.
-
Ritonavir is the best alternative to ketoconazole as an index inhibitor of cytochrome P450-3A in drug-drug interaction studies.Br J Clin Pharmacol. 2015 Sep;80(3):342-50. doi: 10.1111/bcp.12668. Epub 2015 Jun 1. Br J Clin Pharmacol. 2015. PMID: 25923589 Free PMC article. Review.
Cited by
-
A Review on New Frontiers in Drug-Drug Interaction Predictions and Safety Evaluations with In Vitro Cellular Models.Pharmaceutics. 2025 Jun 6;17(6):747. doi: 10.3390/pharmaceutics17060747. Pharmaceutics. 2025. PMID: 40574059 Free PMC article. Review.
-
Paclitaxel, Imatinib and 5-Fluorouracil Increase the Unbound Fraction of Flucloxacillin In Vitro.Antibiotics (Basel). 2020 Jun 8;9(6):309. doi: 10.3390/antibiotics9060309. Antibiotics (Basel). 2020. PMID: 32521723 Free PMC article.
-
Factors Influencing the Steady-State Plasma Concentration of Imatinib Mesylate in Patients With Gastrointestinal Stromal Tumors and Chronic Myeloid Leukemia.Front Pharmacol. 2020 Nov 25;11:569843. doi: 10.3389/fphar.2020.569843. eCollection 2020. Front Pharmacol. 2020. PMID: 33381028 Free PMC article.
-
Drug-drug interaction study of imatinib and voriconazole in vitro and in vivo.Infect Drug Resist. 2019 Apr 30;12:1021-1027. doi: 10.2147/IDR.S199526. eCollection 2019. Infect Drug Resist. 2019. PMID: 31118708 Free PMC article.
-
A cocktail probe approach to evaluate the effect of hormones on the expression and activity of CYP enzymes in human hepatocytes with conditions simulating late stage of pregnancy.Eur J Clin Pharmacol. 2023 Jun;79(6):815-827. doi: 10.1007/s00228-023-03489-1. Epub 2023 Apr 15. Eur J Clin Pharmacol. 2023. PMID: 37060457 Free PMC article.
References
-
- Malfitano A, Barbaro G, Perretti A, Barbarini G. Human immunodeficiency virus-associated malignancies: a therapeutic update. Curr HIV Res. 2012;10:123–32. - PubMed
-
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CDM, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. - PubMed
-
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

