Pharyngeal Satellite Cells Undergo Myogenesis Under Basal Conditions and Are Required for Pharyngeal Muscle Maintenance
- PMID: 26178867
- PMCID: PMC4713349
- DOI: 10.1002/stem.2098
Pharyngeal Satellite Cells Undergo Myogenesis Under Basal Conditions and Are Required for Pharyngeal Muscle Maintenance
Abstract
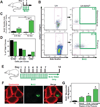
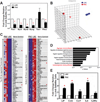
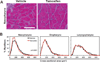
The pharyngeal muscles of the nasal, oral, and laryngeal pharynxes are required for swallowing. Pharyngeal muscles are preferentially affected in some muscular dystrophies yet spared in others. Muscle stem cells, called satellite cells, may be critical factors in the development of pharyngeal muscle disorders; however, very little is known about pharyngeal satellite cells (PSC) and their role in pharyngeal muscles. We show that PSC are distinct from the commonly studied hindlimb satellite cells both transcriptionally and biologically. Under basal conditions PSC proliferate, progress through myogenesis, and fuse with pharyngeal myofibers. Furthermore, PSC exhibit biologic differences dependent on anatomic location in the pharynx. Importantly, PSC are required to maintain myofiber size and myonuclear number in pharyngeal myofibers. Together, these results demonstrate that PSC are critical for pharyngeal muscle maintenance and suggest that satellite cell impairment could contribute to pharyngeal muscle pathology associated with various muscular dystrophies and aging.
Keywords: Muscle maintenance; Myofiber; Myonuclear turnover; Pharynx; Satellite cells.
© 2015 AlphaMed Press.
Conflict of interest statement
The authors report no potential conflicts of interest.
Figures



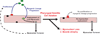
Similar articles
-
Role of satellite cells in muscle growth and maintenance of muscle mass.Nutr Metab Cardiovasc Dis. 2013 Dec;23 Suppl 1:S12-8. doi: 10.1016/j.numecd.2012.02.002. Epub 2012 May 22. Nutr Metab Cardiovasc Dis. 2013. PMID: 22621743 Review.
-
M-cadherin-mediated intercellular interactions activate satellite cell division.J Cell Sci. 2013 Nov 15;126(Pt 22):5116-31. doi: 10.1242/jcs.123562. Epub 2013 Sep 17. J Cell Sci. 2013. PMID: 24046443
-
Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers.Dev Dyn. 2006 Jan;235(1):203-12. doi: 10.1002/dvdy.20602. Dev Dyn. 2006. PMID: 16258933
-
Inactivation of PPARβ/δ adversely affects satellite cells and reduces postnatal myogenesis.Am J Physiol Endocrinol Metab. 2015 Jul 15;309(2):E122-31. doi: 10.1152/ajpendo.00586.2014. Epub 2015 Apr 28. Am J Physiol Endocrinol Metab. 2015. PMID: 25921579
-
The skeletal muscle satellite cell: the stem cell that came in from the cold.J Histochem Cytochem. 2006 Nov;54(11):1177-91. doi: 10.1369/jhc.6R6995.2006. Epub 2006 Aug 9. J Histochem Cytochem. 2006. PMID: 16899758 Review.
Cited by
-
Genome Editing and Muscle Stem Cells as a Therapeutic Tool for Muscular Dystrophies.Curr Stem Cell Rep. 2017;3(2):137-148. doi: 10.1007/s40778-017-0076-6. Epub 2017 Apr 24. Curr Stem Cell Rep. 2017. PMID: 28616376 Free PMC article. Review.
-
A muscle stem cell for every muscle: variability of satellite cell biology among different muscle groups.Front Aging Neurosci. 2015 Oct 7;7:190. doi: 10.3389/fnagi.2015.00190. eCollection 2015. Front Aging Neurosci. 2015. PMID: 26500547 Free PMC article. Review.
-
All for One and One for All: Regenerating Skeletal Muscle.Cold Spring Harb Perspect Biol. 2022 Aug 1;14(8):a040824. doi: 10.1101/cshperspect.a040824. Cold Spring Harb Perspect Biol. 2022. PMID: 34750174 Free PMC article. Review.
-
Distinct Embryonic Origin and Injury Response of Resident Stem Cells in Craniofacial Muscles.Front Physiol. 2021 Jul 1;12:690248. doi: 10.3389/fphys.2021.690248. eCollection 2021. Front Physiol. 2021. PMID: 34276411 Free PMC article. Review.
-
Generation of craniofacial myogenic progenitor cells from human induced pluripotent stem cells for skeletal muscle tissue regeneration.Biomaterials. 2020 Jul;248:119995. doi: 10.1016/j.biomaterials.2020.119995. Epub 2020 Apr 2. Biomaterials. 2020. PMID: 32283390 Free PMC article.
References
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Monaco AP, Bertelson CJ, Liechti-Gallati S, et al. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. - PubMed
-
- Nonaka I. Distal myopathies. Current opinion in neurology. 1999;12:493–499. - PubMed
-
- Bione S, Maestrini E, Rivella S, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994;8:323–327. - PubMed
-
- Bonne G, Di Barletta MR, Varnous S, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

