Efficacy of prednisone for the treatment of ocular myasthenia (EPITOME): A randomized, controlled trial
- PMID: 26179124
- PMCID: PMC6038933
- DOI: 10.1002/mus.24769
Efficacy of prednisone for the treatment of ocular myasthenia (EPITOME): A randomized, controlled trial
Abstract
Introduction: In this study we evaluated the safety, tolerability, and efficacy of prednisone in patients with ocular myasthenia gravis (OMG) concurrently treated with pyridostigmine.
Methods: This investigation was a randomized, double-blind, placebo-controlled trial. Participants whose symptoms failed to remit on pyridostigmine were randomized to receive placebo or prednisone, initiated at 10 mg every other day, and titrated to a maximum of 40 mg/day over 16 weeks. The primary outcome measure was treatment failure.
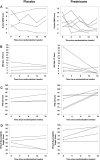
Results: Fewer subjects were randomized than the 88 planned. Of the 11 randomized, 9 completed 16 weeks of double-blind therapy. Treatment failure incidence was 100% (95% CI 48%-100%) in the placebo group (n = 5) vs. 17% (95% CI 0%-64%) in the prednisone group, P = 0.02 (n = 6). Median time to sustained minimal manifestation status (MMS) was 14 weeks, requiring an average prednisone dose of 15 mg/day. Adverse events were infrequent and generally mild in both groups.
Conclusions: A strategy of low-dose prednisone with gradual escalation appears to be safe, well-tolerated, and effective in treating OMG.
Keywords: clinical trial; neuromuscular; ocular myasthenia; prednisone; steroids.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
Conflicts of Interest
None
Figures
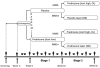
 indicates in-person visit;
indicates in-person visit;
 indicates telephone visit.
indicates telephone visit.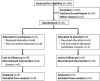
References
-
- Ferguson FR, Hutchinson EC, Liversedge LA. Myasthenia gravis; results of medical management. Lancet. 1955;269:636–639. - PubMed
-
- Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle & nerve. 2008;37:141–149. - PubMed
-
- Sanders D. Approach to Diseases of the Neuromuscular Junction. In: Tawil R, Venance S, editors. Neuromuscular Disorders. First. Vol. 2011. John Wiley & Sons Ltd; pp. 113–117.
-
- Aiello I, Pastorino M, Sotgiu S, et al. Epidemiology of myasthenia gravis in northwestern Sardinia. Neuroepidemiology. 1997;16:199–206. - PubMed
-
- Benatar M, Kaminski H. Medical and surgical treatments for ocular myasthenia. Cochrane Database Syst Rev. 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

