Effects of different antibiotic classes on airway bacteria in stable COPD using culture and molecular techniques: a randomised controlled trial
- PMID: 26179246
- PMCID: PMC4602260
- DOI: 10.1136/thoraxjnl-2015-207194
Effects of different antibiotic classes on airway bacteria in stable COPD using culture and molecular techniques: a randomised controlled trial
Abstract
Background: Long-term antibiotic therapy is used to prevent exacerbations of COPD but there is uncertainty over whether this reduces airway bacteria. The optimum antibiotic choice remains unknown. We conducted an exploratory trial in stable patients with COPD comparing three antibiotic regimens against placebo.
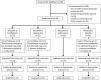
Methods: This was a single-centre, single-blind, randomised placebo-controlled trial. Patients aged ≥45 years with COPD, FEV1<80% predicted and chronic productive cough were randomised to receive either moxifloxacin 400 mg daily for 5 days every 4 weeks, doxycycline 100 mg/day, azithromycin 250 mg 3 times a week or one placebo tablet daily for 13 weeks. The primary outcome was the change in total cultured bacterial load in sputum from baseline; secondary outcomes included bacterial load by 16S quantitative PCR (qPCR), sputum inflammation and antibiotic resistance.
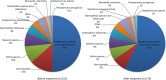
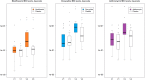
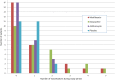
Results: 99 patients were randomised; 86 completed follow-up, were able to expectorate sputum and were analysed. After adjustment, there was a non-significant reduction in bacterial load of 0.42 log10 cfu/mL (95% CI -0.08 to 0.91, p=0.10) with moxifloxacin, 0.11 (-0.33 to 0.55, p=0.62) with doxycycline and 0.08 (-0.38 to 0.54, p=0.73) with azithromycin from placebo, respectively. There were also no significant changes in bacterial load measured by 16S qPCR or in airway inflammation. More treatment-related adverse events occurred with moxifloxacin. Of note, mean inhibitory concentrations of cultured isolates increased by at least three times over placebo in all treatment arms.
Conclusions: Total airway bacterial load did not decrease significantly after 3 months of antibiotic therapy. Large increases in antibiotic resistance were seen in all treatment groups and this has important implications for future studies.
Trial registration number: clinicaltrials.gov (NCT01398072).
Keywords: COPD Exacerbations; COPD Pathology; Respiratory Infection.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
