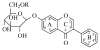Interaction of Isoflavones with the BCRP/ABCG2 Drug Transporter
- PMID: 26179608
- PMCID: PMC4713194
- DOI: 10.2174/138920021602150713114921
Interaction of Isoflavones with the BCRP/ABCG2 Drug Transporter
Abstract
This review will provide a comprehensive overview of the interactions between dietary isoflavones and the ATP-binding cassette (ABC) G2 efflux transporter, which is also named the breast cancer resistance protein (BCRP). Expressed in a variety of organs including the liver, kidneys, intestine, and placenta, BCRP mediates the disposition and excretion of numerous endogenous chemicals and xenobiotics. Isoflavones are a class of naturallyoccurring compounds that are found at high concentrations in commonly consumed foods and dietary supplements. A number of isoflavones, including genistein and daidzein and their metabolites, interact with BCRP as substrates, inhibitors, and/or modulators of gene expression. To date, a variety of model systems have been employed to study the ability of isoflavones to serve as substrates and inhibitors of BCRP; these include whole cells, inverted plasma membrane vesicles, in situ organ perfusion, as well as in vivo rodent and sheep models. Evidence suggests that BCRP plays a role in mediating the disposition of isoflavones and in particular, their conjugated forms. Furthermore, as inhibitors, these compounds may aid in reversing multidrug resistance and sensitizing cancer cells to chemotherapeutic drugs. This review will also highlight the consequences of altered BCRP expression and/or function on the pharmacokinetics and toxicity of chemicals following isoflavone exposure.
Conflict of interest statement
The authors declare no conflicts of interest. This work was supported by the National Institutes of Environmental Health Sciences (Grants ES020522, ES005022, ES007148, ES021800, DK093903), a component of the National Institutes of Health. Kristin Bircsak is supported by predoctoral fellowships from the American Foundation for Pharmaceutical Education and Pharmaceutical Research and Manufacturers of America.
Figures
References
-
- U.S. Department of Agriculture, Agricultural Research Service. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0. Nutrient Data Laboratory; 2008. [Accessed August 13, 2013]. Home Page: http://www.ars.usda.gov/nutrientdata/isoflav.
-
- Rolfe BG. Flavones and isoflavones as inducing substances of legume nodulation. BioFactors. 1988;1:3–10. - PubMed
-
- Burdette CQ, Marcus RK. Determination of isoflavone content in soy, red clover, and kudzu dietary supplement materials by liquid chromatography-particle beam/electron ionization mass spectrometry. J AOAC Int. 2013;96:925–32. - PubMed
-
- Song T, Barua K, Buseman G, Murphy PA. Soy isoflavone analysis: quality control and a new internal standard. Am J Clin Nutr. 1998;68:1474S–1479S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources