Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety
- PMID: 26179626
- PMCID: PMC4518290
- DOI: 10.1038/ncomms8660
Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety
Erratum in
-
Corrigendum: Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety.Nat Commun. 2016 Oct 10;7:12705. doi: 10.1038/ncomms12705. Nat Commun. 2016. PMID: 27721478 Free PMC article. No abstract available.
Abstract
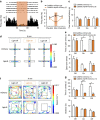
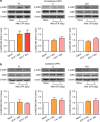
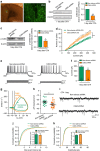
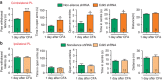
The medial prefrontal cortex (mPFC) is implicated in processing sensory-discriminative and affective pain. Nonetheless, the underlying mechanisms are poorly understood. Here we demonstrate a role for excitatory neurons in the prelimbic cortex (PL), a sub-region of mPFC, in the regulation of pain sensation and anxiety-like behaviours. Using a chronic inflammatory pain model, we show that lesion of the PL contralateral but not ipsilateral to the inflamed paw attenuates hyperalgesia and anxiety-like behaviours in rats. Optogenetic activation of contralateral PL excitatory neurons exerts analgesic and anxiolytic effects in mice subjected to chronic pain, whereas inhibition is anxiogenic in naive mice. The intrinsic excitability of contralateral PL excitatory neurons is decreased in chronic pain rats; knocking down cyclin-dependent kinase 5 reverses this deactivation and alleviates behavioural impairments. Together, our findings provide novel insights into the role of PL excitatory neurons in the regulation of sensory and affective pain.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Acute pharmacogenetic activation of medial prefrontal cortex excitatory neurons regulates anxiety-like behaviour.J Biosci. 2018 Mar;43(1):85-95. J Biosci. 2018. PMID: 29485117
-
Altered Excitability and Local Connectivity of mPFC-PAG Neurons in a Mouse Model of Neuropathic Pain.J Neurosci. 2018 May 16;38(20):4829-4839. doi: 10.1523/JNEUROSCI.2731-17.2018. Epub 2018 Apr 25. J Neurosci. 2018. PMID: 29695413 Free PMC article.
-
Oxytocin in the medial prefrontal cortex attenuates anxiety: Anatomical and receptor specificity and mechanism of action.Neuropharmacology. 2017 Oct;125:1-12. doi: 10.1016/j.neuropharm.2017.06.024. Epub 2017 Jun 24. Neuropharmacology. 2017. PMID: 28655609 Free PMC article.
-
Differential Rearrangement of Excitatory Inputs to the Medial Prefrontal Cortex in Chronic Pain Models.Front Neural Circuits. 2021 Dec 24;15:791043. doi: 10.3389/fncir.2021.791043. eCollection 2021. Front Neural Circuits. 2021. PMID: 35002635 Free PMC article. Review.
-
[Application of optogenetic technique in pain research].Sheng Li Xue Bao. 2016 Oct 25;68(5):655-660. Sheng Li Xue Bao. 2016. PMID: 27778031 Review. Chinese.
Cited by
-
dmPFC-vlPAG projection neurons contribute to pain threshold maintenance and antianxiety behaviors.J Clin Invest. 2020 Dec 1;130(12):6555-6570. doi: 10.1172/JCI127607. J Clin Invest. 2020. PMID: 32841213 Free PMC article.
-
Local production of corticotropin-releasing hormone in prefrontal cortex modulates male-specific novelty exploration.Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2211454119. doi: 10.1073/pnas.2211454119. Epub 2022 Nov 29. Proc Natl Acad Sci U S A. 2022. PMID: 36442105 Free PMC article.
-
Metabotropic Glutamate Receptor 5 in the Medial Prefrontal Cortex as a Molecular Determinant of Pain and Ensuing Depression.Front Mol Neurosci. 2018 Oct 8;11:376. doi: 10.3389/fnmol.2018.00376. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30349459 Free PMC article.
-
The Role of Mesostriatal Dopamine System and Corticostriatal Glutamatergic Transmission in Chronic Pain.Brain Sci. 2021 Oct 2;11(10):1311. doi: 10.3390/brainsci11101311. Brain Sci. 2021. PMID: 34679376 Free PMC article. Review.
-
The signaling proteins GPR158 and RGS7 modulate excitability of L2/3 pyramidal neurons and control A-type potassium channel in the prelimbic cortex.J Biol Chem. 2019 Aug 30;294(35):13145-13157. doi: 10.1074/jbc.RA119.007533. Epub 2019 Jul 16. J Biol Chem. 2019. PMID: 31311860 Free PMC article.
References
-
- Reid K. J. et al.. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr. Med. Res. Opin. 27, 449–462 (2011) . - PubMed
-
- Price D. D. Psychological and neural mechanisms of the affective dimension of pain. Science 288, 1769–1772 (2000) . - PubMed
-
- Dersh J., Polatin P. B. & Gatchel R. J. Chronic pain and psychopathology: research findings and theoretical considerations. Psychosom. Med. 64, 773–786 (2002) . - PubMed
-
- Bair M. J., Robinson R. L., Katon W. & Kroenke K. Depression and pain comorbidity: a literature review. Arch. Intern. Med. 163, 2433–2445 (2003) . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

