Acylation of Biomolecules in Prokaryotes: a Widespread Strategy for the Control of Biological Function and Metabolic Stress
- PMID: 26179745
- PMCID: PMC4503791
- DOI: 10.1128/MMBR.00020-15
Acylation of Biomolecules in Prokaryotes: a Widespread Strategy for the Control of Biological Function and Metabolic Stress
Abstract
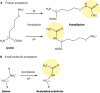
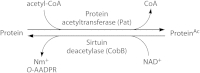
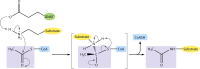
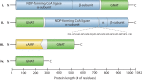
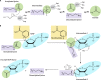
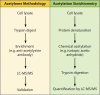
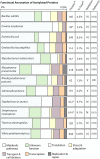
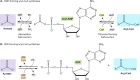
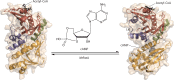
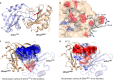
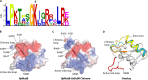
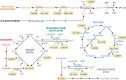
Acylation of biomolecules (e.g., proteins and small molecules) is a process that occurs in cells of all domains of life and has emerged as a critical mechanism for the control of many aspects of cellular physiology, including chromatin maintenance, transcriptional regulation, primary metabolism, cell structure, and likely other cellular processes. Although this review focuses on the use of acetyl moieties to modify a protein or small molecule, it is clear that cells can use many weak organic acids (e.g., short-, medium-, and long-chain mono- and dicarboxylic aliphatics and aromatics) to modify a large suite of targets. Acetylation of biomolecules has been studied for decades within the context of histone-dependent regulation of gene expression and antibiotic resistance. It was not until the early 2000s that the connection between metabolism, physiology, and protein acetylation was reported. This was the first instance of a metabolic enzyme (acetyl coenzyme A [acetyl-CoA] synthetase) whose activity was controlled by acetylation via a regulatory system responsive to physiological cues. The above-mentioned system was comprised of an acyltransferase and a partner deacylase. Given the reversibility of the acylation process, this system is also referred to as reversible lysine acylation (RLA). A wealth of information has been obtained since the discovery of RLA in prokaryotes, and we are just beginning to visualize the extent of the impact that this regulatory system has on cell function.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

