Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections
- PMID: 26180063
- PMCID: PMC4503790
- DOI: 10.1128/CMR.00002-15
Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections
Abstract
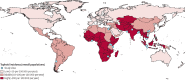
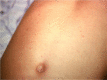
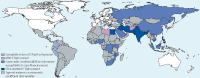
Salmonella enterica infections are common causes of bloodstream infection in low-resource areas, where they may be difficult to distinguish from other febrile illnesses and may be associated with a high case fatality ratio. Microbiologic culture of blood or bone marrow remains the mainstay of laboratory diagnosis. Antimicrobial resistance has emerged in Salmonella enterica, initially to the traditional first-line drugs chloramphenicol, ampicillin, and trimethoprim-sulfamethoxazole. Decreased fluoroquinolone susceptibility and then fluoroquinolone resistance have developed in association with chromosomal mutations in the quinolone resistance-determining region of genes encoding DNA gyrase and topoisomerase IV and also by plasmid-mediated resistance mechanisms. Resistance to extended-spectrum cephalosporins has occurred more often in nontyphoidal than in typhoidal Salmonella strains. Azithromycin is effective for the management of uncomplicated typhoid fever and may serve as an alternative oral drug in areas where fluoroquinolone resistance is common. In 2013, CLSI lowered the ciprofloxacin susceptibility breakpoints to account for accumulating clinical, microbiologic, and pharmacokinetic-pharmacodynamic data suggesting that revision was needed for contemporary invasive Salmonella infections. Newly established CLSI guidelines for azithromycin and Salmonella enterica serovar Typhi were published in CLSI document M100 in 2015.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, et al. . 2012. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2197–2223. doi:10.1016/S0140-6736(12)61689-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 TW009237/TW/FIC NIH HHS/United States
- BB/L018845/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L017679/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L018845/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J010367/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

