Purification and Characterization of a Polyextremophilic α -Amylase from an Obligate Halophilic Aspergillus penicillioides Isolate and Its Potential for Souse with Detergents
- PMID: 26180787
- PMCID: PMC4477103
- DOI: 10.1155/2015/245649
Purification and Characterization of a Polyextremophilic α -Amylase from an Obligate Halophilic Aspergillus penicillioides Isolate and Its Potential for Souse with Detergents
Abstract
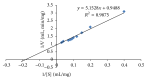
An extracellular α-amylase from the obligate halophilic Aspergillus penicillioides TISTR3639 strain was produced and enriched to apparent homogeneity by ammonium sulfate precipitation and Sephadex G100 gel filtration column chromatography. The mass of the purified amylase was estimated to be 42 kDa by SDS-PAGE. With soluble starch as the substrate it had a specific activity of 118.42 U · mg(-1) and Vmax and Km values of 1.05 µmol · min(-1) · mg(-1) and 5.41 mg · mL(-1), respectively. The enzyme was found to have certain polyextremophilic characteristics, with an optimum activity at pH 9, 80 °C, and 300 g · L(-1) NaCl. The addition of CaCl2 at 2 mM was found to slightly enhance the amylase activity, while ZnCl2, FeCl2, or EDTA at 2 mM was strongly or moderately inhibitory, respectively, suggesting the requirement for a (non-Fe(2+) or Zn(2+)) divalent cation. The enzyme retained more than 80% of its activity when incubated with three different laundry detergents and had a better performance compared to a commercial amylase and three detergents in the presence of increasing NaCl concentrations up to 300 g · L(-1). Accordingly, it has a good potential for use as an α-amylase in a low water activity (high salt concentration) and at high pH and temperatures.
Figures

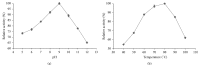


References
-
- Ali I., Kanhayuwa L., Rachdawong S., Rakshit S. K. Identification, phylogenetic analysis and characterization of obligate halophilic fungi isolated from a man-made solar saltern in Phetchaburi province, Thailand. Annals of Microbiology. 2013;63(3):887–895. doi: 10.1007/s13213-012-0540-6. - DOI
-
- Gunde-Cimerman N., Frisvad J. C., Zalaretal P. Halotolerant and halophilic fungi. In: Deshmukh S. K., Rai M. K., editors. Biodiversity of Fungi-Their Role in Human Life. New Delhi, India: Oxford & IBH; 2005. pp. 96–128.
-
- Ali I., Siwarungson N., Punnapayak H., et al. Screening of potential biotechnological applications from obligate halophilic fungi, isolated from a man-made solar saltern located in Phetchaburi province, Thailand. Pakistan Journal of Botany. 2014;46:983–988.
-
- Ali I., Rakshit S. K., Akbar A., et al. Identification and phylogenetic analysis of halophilic fungus isolated from a man-made solar saltern in Thailand. Lasbella University Journal of Science and Technology. 2013;2:47–52.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

