Hemagglutination Inhibition Antibody Titers as a Correlate of Protection Against Seasonal A/H3N2 Influenza Disease
- PMID: 26180823
- PMCID: PMC4498268
- DOI: 10.1093/ofid/ofv067
Hemagglutination Inhibition Antibody Titers as a Correlate of Protection Against Seasonal A/H3N2 Influenza Disease
Abstract
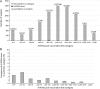
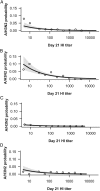
Background. To investigate the relationship between hemagglutinin-inhibition (HI) antibody levels to the risk of influenza disease, we conducted a correlate of protection analysis using pooled data from previously published randomized trials. Methods. Data on the occurrence of laboratory-confirmed influenza and HI levels pre- and postvaccination were analyzed from 4 datasets: 3 datasets included subjects aged <65 years who received inactivated trivalent influenza vaccine (TIV) or placebo, and 1 dataset included subjects aged ≥65 years who received AS03-adjuvanted TIV (AS03-TIV) or TIV. A logistic model was used to evaluate the relationship between the postvaccination titer of A/H3N2 HI antibodies and occurrence of A/H3N2 disease. We then built a receiver-operating characteristic curve to identify a potential cutoff titer between protection and no protection. Results. The baseline odds ratio of A/H3N2 disease was higher for subjects aged ≥65 years than <65 years and higher in seasons of strong epidemic intensity than moderate or low intensity. Including age and epidemic intensity as covariates, a 4-fold increase in titer was associated with a 2-fold decrease in the risk of A/H3N2 disease. Conclusions. The modeling exercise confirmed a relationship between A/H3N2 disease and HI responses, but it did not allow an evaluation of the predictive power of the HI response.
Keywords: A/H3N2; influenza; modeling; serologic correlates; vaccine.
Figures

References
-
- Barrett PN, Berezuk G, Fritsch S et al. . Efficacy, safety, and immunogenicity of a Vero-cell-culture-derived trivalent influenza vaccine: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2011; 377:751–9. - PubMed
-
- Black S, Nicolay U, Vesikari T et al. . Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081–5. - PubMed
-
- Siber GR. Methods for estimating serological correlates of protection. Dev Biol Stand 1997; 89:283–96. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

