Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1
- PMID: 26181576
- PMCID: PMC4589106
- DOI: 10.1089/ars.2015.6385
Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1
Abstract
Significance: Protein structure and function can be regulated via post-translational modifications by numerous enzymatic and nonenzymatic mechanisms. Regulation involving oxidation of sulfur-containing residues emerged as a key mechanism of redox control. Unraveling the participants and principles of such regulation is necessary for understanding the biological significance of redox control of cellular processes.
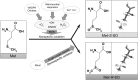
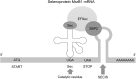
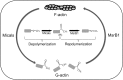
Recent advances: Reversible oxidation of methionine residues by monooxygenases of the Mical family and subsequent reduction of methionine sulfoxides by a selenocysteine-containing methionine sulfoxide reductase B1 (MsrB1) was found to control the assembly and disassembly of actin in mammals, and the Mical/MsrB pair similarly regulates actin in fruit flies. This finding has opened up new avenues for understanding the use of stereospecific methionine oxidation in regulating cellular processes and the roles of MsrB1 and Micals in regulation of actin dynamics.
Critical issues: So far, Micals have been the only known partners of MsrB1, and actin is the only target. It is important to identify additional substrates of Micals and characterize other Mical-like enzymes.
Future directions: Oxidation of methionine, reviewed here, is an emerging but not well-established mechanism. Studies suggest that methionine oxidation is a form of oxidative damage of proteins, a modification that alters protein structure or function, a tool in redox signaling, and a mechanism that controls protein function. Understanding the functional impact of reversible oxidation of methionine will require identification of targets, substrates, and regulators of Micals and Msrs. Linking the biological processes, in which these proteins participate, might also lead to insights into disease conditions, which involve regulation of actin by Micals and Msrs.
Figures

Similar articles
-
Regulated methionine oxidation by monooxygenases.Free Radic Biol Med. 2017 Aug;109:141-155. doi: 10.1016/j.freeradbiomed.2017.02.010. Epub 2017 Feb 14. Free Radic Biol Med. 2017. PMID: 28229915 Free PMC article. Review.
-
MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation.Mol Cell. 2013 Aug 8;51(3):397-404. doi: 10.1016/j.molcel.2013.06.019. Epub 2013 Aug 1. Mol Cell. 2013. PMID: 23911929 Free PMC article.
-
The selenoprotein methionine sulfoxide reductase B1 (MSRB1).Free Radic Biol Med. 2022 Oct;191:228-240. doi: 10.1016/j.freeradbiomed.2022.08.043. Epub 2022 Sep 7. Free Radic Biol Med. 2022. PMID: 36084791 Review.
-
The methionine sulfoxide reduction system: selenium utilization and methionine sulfoxide reductase enzymes and their functions.Antioxid Redox Signal. 2013 Sep 20;19(9):958-69. doi: 10.1089/ars.2012.5081. Epub 2013 Jan 22. Antioxid Redox Signal. 2013. PMID: 23198996 Free PMC article. Review.
-
Selenium and Methionine Sulfoxide Reduction.Free Radic Biol Med. 2014 Oct;75 Suppl 1:S8-9. doi: 10.1016/j.freeradbiomed.2014.10.848. Epub 2014 Dec 10. Free Radic Biol Med. 2014. PMID: 26461418
Cited by
-
Regulation of Retroviral and SARS-CoV-2 Protease Dimerization and Activity through Reversible Oxidation.Antioxidants (Basel). 2022 Oct 18;11(10):2054. doi: 10.3390/antiox11102054. Antioxidants (Basel). 2022. PMID: 36290777 Free PMC article. Review.
-
A Vitamin B2-Photocatalysed Approach to Methionine Analogues.Angew Chem Weinheim Bergstr Ger. 2022 Dec 12;134(50):e202212158. doi: 10.1002/ange.202212158. Epub 2022 Nov 10. Angew Chem Weinheim Bergstr Ger. 2022. PMID: 38505624 Free PMC article.
-
Selenium, a Micronutrient That Modulates Cardiovascular Health via Redox Enzymology.Nutrients. 2021 Sep 17;13(9):3238. doi: 10.3390/nu13093238. Nutrients. 2021. PMID: 34579115 Free PMC article. Review.
-
A peptide-crosslinking approach identifies HSPA8 and PFKL as selective interactors of an actin-derived peptide containing reduced and oxidized methionine.RSC Chem Biol. 2022 Sep 15;3(10):1282-1289. doi: 10.1039/d2cb00183g. eCollection 2022 Oct 5. RSC Chem Biol. 2022. PMID: 36320891 Free PMC article.
-
Features and regulation of non-enzymatic post-translational modifications.Nat Chem Biol. 2018 Feb 14;14(3):244-252. doi: 10.1038/nchembio.2575. Nat Chem Biol. 2018. PMID: 29443975 Review.
References
-
- Bigelow DJ. and Squier TC. Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochim Biophys Acta 1703: 121–134, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

