Implementation and Operational Research: Integration of PMTCT and Antenatal Services Improves Combination Antiretroviral Therapy Uptake for HIV-Positive Pregnant Women in Southern Zambia: A Prototype for Option B+?
- PMID: 26181813
- PMCID: PMC6754251
- DOI: 10.1097/QAI.0000000000000760
Implementation and Operational Research: Integration of PMTCT and Antenatal Services Improves Combination Antiretroviral Therapy Uptake for HIV-Positive Pregnant Women in Southern Zambia: A Prototype for Option B+?
Abstract
Background: Early initiation of combination antiretroviral therapy (cART) for HIV-positive pregnant women can decrease vertical transmission to less than 5%. Programmatic barriers to early cART include decentralized care, disease-stage assessment delays, and loss to follow-up.
Intervention: Our intervention had 3 components: integrated HIV and antenatal services in 1 location with 1 provider, laboratory courier to expedite CD4 counts, and community-based follow-up of women-infant pairs to improve prevention of mother-to-child transmission attendance. Preintervention HIV-positive pregnant women were referred to HIV clinics for disease-stage assessment and cART initiation for advanced disease (CD4 count <350 cells/μL or WHO stage >2).
Methods: We used a quasi-experimental design with preintervention/postintervention evaluations at 6 government antenatal clinics (ANCs) in Southern Province, Zambia. Retrospective clinical data were collected from clinic registers during a 7-month baseline period. Postintervention data were collected from all antiretroviral therapy-naive, HIV-positive pregnant women and their infants presenting to ANC from December 2011 to June 2013.
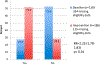
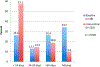
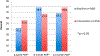
Results: Data from 510 baseline women-infant pairs were analyzed and 624 pregnant women were enrolled during the intervention period. The proportion of HIV-positive pregnant women receiving CD4 counts increased from 50.6% to 77.2% [relative risk (RR) = 1.81; 95% confidence interval (CI): 1.57 to 2.08; P < 0.01]. The proportion of cART-eligible pregnant women initiated on cART increased from 27.5% to 71.5% (RR = 2.25; 95% CI: 1.78 to 2.83; P < 0.01). The proportion of eligible HIV-exposed infants with documented 6-week HIV PCR test increased from 41.9% to 55.8% (RR = 1.33; 95% CI: 1.18 to 1.51; P < 0.01).
Conclusions: Integration of HIV care into ANC and community-based support improved uptake of CD4 counts, proportion of cART-eligible women initiated on cART, and infants tested.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–180. Available at: http://www.sciencedirect.com/science/article/pii/S1473309910702887. Accessed October 2, 2013. - PubMed
-
- World Health Organization; Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Recommendations for a Public Health Approach. Geneva, Switzerland, WHO Press; 2010. - PubMed
-
- Danel C, Moh R, Minga A, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in West Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367: 1981–1989. - PubMed
-
- Danel C, Moh R, Chaix ML, et al. Two-months-off, four-months-on antiretroviral regimen increases the risk of resistance, compared with continuous therapy: a randomized trial involving West African adults. J Infect Dis. 2009;199:66–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

