Vaccination Drives Changes in Metabolic and Virulence Profiles of Streptococcus pneumoniae
- PMID: 26181911
- PMCID: PMC4504489
- DOI: 10.1371/journal.ppat.1005034
Vaccination Drives Changes in Metabolic and Virulence Profiles of Streptococcus pneumoniae
Abstract
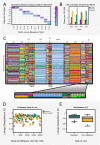
The bacterial pathogen, Streptococcus pneumoniae (the pneumococcus), is a leading cause of life-threatening illness and death worldwide. Available conjugate vaccines target only a small subset (up to 13) of >90 known capsular serotypes of S. pneumoniae and, since their introduction, increases in non-vaccine serotypes have been recorded in several countries: a phenomenon termed Vaccine Induced Serotype Replacement (VISR). Here, using a combination of mathematical modelling and whole genome analysis, we show that targeting particular serotypes through vaccination can also cause their metabolic and virulence-associated components to transfer through recombination to non-vaccine serotypes: a phenomenon we term Vaccine-Induced Metabolic Shift (VIMS). Our results provide a novel explanation for changes observed in the population structure of the pneumococcus following vaccination, and have important implications for strain-targeted vaccination in a range of infectious disease systems.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5(2):83–93. - PubMed
-
- Maiden MCJ, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, et al. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(6):3140–5. - PMC - PubMed
-
- Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. Journal of Infectious Diseases. 2003;187(9):1424–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

