Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- PMID: 26182347
- PMCID: PMC4504509
- DOI: 10.1371/journal.ppat.1005044
Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
Abstract
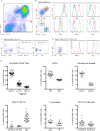
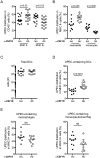
Urinary tract infection (UTI) is one of the most common bacterial infections with frequent recurrence being a major medical challenge. Development of effective therapies has been impeded by the lack of knowledge of events leading to adaptive immunity. Here, we establish conclusive evidence that an adaptive immune response is generated during UTI, yet this response does not establish sterilizing immunity. To investigate the underlying deficiency, we delineated the naïve bladder immune cell compartment, identifying resident macrophages as the most populous immune cell. To evaluate their impact on the establishment of adaptive immune responses following infection, we measured bacterial clearance in mice depleted of either circulating monocytes, which give rise to macrophages, or bladder resident macrophages. Surprisingly, mice depleted of resident macrophages, prior to primary infection, exhibited a nearly 2-log reduction in bacterial burden following secondary challenge compared to untreated animals. This increased bacterial clearance, in the context of a challenge infection, was dependent on lymphocytes. Macrophages were the predominant antigen presenting cell to acquire bacteria post-infection and in their absence, bacterial uptake by dendritic cells was increased almost 2-fold. These data suggest that bacterial uptake by tissue macrophages impedes development of adaptive immune responses during UTI, revealing a novel target for enhancing host responses to bacterial infection of the bladder.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Russo TA, Johnson JR (2003) Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect 5: 449–456. - PubMed
-
- Johnson CC (1991) Definitions, classification, and clinical presentation of urinary tract infections. Med Clin North Am 75: 241–252. - PubMed
-
- Anderson GG, Dodson KW, Hooton TM, Hultgren SJ (2004) Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol 12: 424–430. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

